Abstract
Rationale
The clinical characteristics and prognostic impact of radiographic patterns of patients with nontuberculous mycobacterial lung disease (NTM-LD) are rarely evaluated.
Design
Patients with NTM-LD from 2007 to 2009 in a single medical center in Taiwan were identified. Their radiographic patterns were reviewed and classified into cavitary, bronchiectatic, or consolidative. They were also compared to patients with cavitary pulmonary tuberculosis (TB-LD).
Results
Of 481 NTM-LD patients identified, 62, 134, and 56 patients were categorized into cavitary, bronchiectatic, and consolidative groups, respectively. Compared with 180 TB-LD patients, cavitary NTM-LD had male predominance and was associated with higher grades of sputum acid-fast smear (3+ or 4+), prior pulmonary TB, and poor baseline pulmonary function. NTM-LD patients with consolidative pattern were likely to have underlying comorbidity, the highest blood leukocyte count and C-reactive protein, and lowest albumin. In all NTM-LD, the consolidative pattern was independently associated with poor prognosis for 6-month survival. Patients with cavitary Mycobacterium avium complex (MAC)-LD had worse 6-month survival than those with bronchiectatic pattern.
Conclusion
In Taiwan, NTM-LD patients with consolidative pattern have the worst prognosis while patients with cavitary pattern have worse survival than those with bronchiectasis in MAC-LD. Because varying radiographic patterns represent different prognoses, understanding the characteristics of NTM-LD patients with different radiographic patterns complements clinical practice.
Similar content being viewed by others
Introduction
Nontuberculous mycobacteria (NTM) are an important clinical concern because the incidence of NTM lung disease (NTM-LD) has increased over the past 10 years [1–3]. The multifactorial causes include decreased cross-protection from TB, increasing population with acquired immunocompromised conditions, and advances in mycobacterial culture [4–7]. NTM-LD is traditionally considered an indolent process and diagnosis is based on the multiple criteria established by the American Thoracic Society (ATS) [3]. Of these criteria, the microbiological criterion is dependent on a time-consuming mycobacterial culture in two or more respiratory samples. In contrast, chest radiography is the initial tool for a complementary approach because of its great availability and short examination time [8].
Typical radiographic findings of NTM-LD include fibrocavitary lesions, nodules, and bronchiectasis [3]. Approximately 40–50% of smear-positive and 20% of smear-negative cases belong to the fibrocavitary group [9, 10]. A previous report on Mycobacterium avium complex (MAC) lung disease suggests that patients with cavitary lesions may have worse prognosis than those without [11]. However, such findings have rarely been investigated in lung disease due to other NTM species [9]. Moreover, the consolidative pattern, an unusual radiographic finding, is frequently seen in NTM-LD patients admitted into intensive care units and may be associated with poor prognosis [6].
It is important to understand the prognostic impact of radiographic patterns on NTM-LD patients because the aggressiveness of treatment may be different. This retrospective review in a referral center in Taiwan aimed to compare the clinical characteristics, laboratory findings, and outcomes of NTM-LD patients based on their radiographic patterns.
Materials and Methods
This study was conducted in National Taiwan University Hospital, a tertiary referral center in northern Taiwan. The medical records and mycobacterial laboratory registry database were reviewed to identify all respiratory samples sent for mycobacterial culture from January 2007 to December 2009. Mycobacterial culture was performed as previously described [12] and the Mycobacterium species were identified using conventional biochemical testing [13]. The Ethics Committee of the hospital’s Institutional Review Board of Research approved the study (ID: 201009046R).
Patients with respiratory samples yielding Mycobacterium were identified. NTM-LD was diagnosed if all the following were met (guidelines by the ATS) [3]: (1) at least two sputum or one bronchial washing/brushing sample, or one lung tissue culture-positive for the same NTM species; (2) presence of respiratory symptoms; (3) chest radiography or computed tomography (CT) demonstrating new patch(es) of consolidation, exudative, nodular infiltrates, cavitary lesions, or multifocal bronchiectasis; and (4) exclusion of other pulmonary causes. The first NTM culture-positive respiratory sample was the index sample. Pulmonary tuberculosis (TB) was diagnosed based on results of mycobacterial culture of respiratory samples [14].
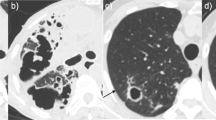
Two pulmonary specialists (CCS and JYW) independently reviewed the chest-imaging studies. If there was a discrepancy in interpretations, the image was further reviewed by a pulmonary specialist blinded to the results. Patterns of the main lesion (>50%) were categorized into cavitary, bronchiectatic, consolidative, and others (e.g. fibroexudative lesion, nodule, mass, and pleurisy), but only patients with the first three radiographic patterns were included for further analysis. The main radiographic lesion consisting of cavitations was defined as cavitary pattern [15]. Lesions were classified as consolidative pattern if there were alveolar opacities and as bronchiectatic pattern if there were radiographic signs of bronchiectasis, including tram tracks or ring shadows [16, 17]. Patients with cavitary pulmonary TB diagnosed during the study period were selected for comparison. The presence of pulmonary fibrosis with decreased lung volume was defined as air-space volume reduction.
Clinical data, including age, gender, underlying comorbidities, initial symptoms, history of pulmonary TB, and laboratory data on the date when the index sample was collected, as well as antimycobacterial treatment and outcome, were recorded. Pulmonary function testing was performed as previously described [18]. Pulmonary function data within 3 months before the index sample as taken were collected and interpreted according to guidelines established by the ATS/European Respiratory Society [19]. The adequacy of anti-NTM and antituberculous treatment was also determined according to the ATS guidelines [3, 14]. Patients were followed up for at least 6 months after the index culture or until death or loss of follow-up.
Statistical Analysis
Intergroup differences were compared by one-way ANOVA for numerical variables and χ2 test for categorical variables. Survival curves for each class of variables were generated using the Kaplan–Meier method and compared using the log-rank test. Multivariate Cox proportional hazard regression analyses were used to identify factors associated with 6-month survival. In the stepwise variable selection procedure, all potential predictors were included. Significance levels for entry and stay were set at 0.15, while a two-sided p < 0.05 was considered significant. All analyses were performed using the SPSS v13.0 (SPSS, Inc., Chicago, IL).
Results
During the study period, 4,553 samples from 2,149 patients yielded NTM (Fig. 1). NTM-LD was diagnosed in 481 patients, with 62 (13%), 134 (28%), and 56 (12%) classified as cavitary, bronchiectatic, and consolidative groups, respectively. The radiographic patterns of the remaining 229 patients included 141 fibroexudative, 84 nodular or mass, and 4 pleural changes. Of the 481 patients, 348 (72%) had undergone chest CT scan. Based on these CT findings, 48 were classified into the cavitary group (77% of the 62), 116 into the bronchiectatic group (87% of the 134), and 35 into the consolidative group (63% of the 56). Among 988 culture-confirmed TB patients, 180 with cavitary TB lung disease (TB-LD) were selected for comparison.
Patients with cavitary NTM-LD or cavitary TB-LD were younger and had male predominance (Table 1). More patients in the cavitary NTM-LD group had a prior history of TB. Underlying diseases causing immunocompromise, such as diabetes mellitus, acquired immunodeficiency syndromes, and liver cirrhosis, were more common in the consolidative NTM-LD group. Of TB-LD patients, 32% had underlying disease, with cancer the most common. Cough was the most common symptom except in consolidative NTM-LD, which was more likely to have fever and dyspnea. Patients with cavitary and bronchiectatic NTM-LD suffered from hemoptysis more frequently than the other two groups.
In bronchiectatic or consolidative NTM-LD groups, MAC was the most common species, followed by M. chelonae-abscessus (Table 2), which was the most common species in the cavitary group. Rapidly growing mycobacteria (RGM) accounted for about 40% in every NTM group. M. kansasii seemed more common in NTM patients with cavitary and consolidative patterns than in those with bronchiectatic pattern. More than 60% of cavitary TB-LD and NTM-LD patients were smear-positive.
The extent of lung involvement on chest radiographs was similar in all groups except for middle lung field involvement, which tended to be more common in NTM-LD (Table 2). In terms of laboratory blood test, consolidative NTM-LD had higher leukocyte count and serum C-reactive protein and lower hemoglobin and albumin than the other groups.
Baseline pulmonary function was available in 19 patients with cavitary TB-LD, as well as in 10, 44, and 10 patients with cavitary, bronchiectatic, and consolidative NTM-LD, respectively (Supplementary material). Patients with cavitary NTM-LD had a moderately restrictive ventilatory defect while those with bronchiectatic and consolidative NTM-LD had a mild restrictive defect. Baseline lung function was within normal range in the cavitary TB-LD group.
Antimycobacterial treatment was given to 101 NTM patients, especially those with consolidative NTM-LD. Of them, 39 received empirical anti-TB medication as their initial regimen (Table 3). Among the treated NTM patients, the cavitary and bronchiectatic groups had longer delays in treatment after the index sample was collected. Within 6 months of follow-up, 14 NTM patients died of multiple organ failure and 11 of refractory respiratory failure.
Consolidative NTM-LD had the worst survival (Fig. 2a). There was no significant intergroup survival difference among those with cavitary TB-LD, cavitary NTM-LD, and bronchiectatic NTM-LD (p = 0.112, by log-rank test). Survival was worse in the cavitary group than in the bronchiectatic group in MAC-LD patients (Fig. 2b) but similar to those with M. chelonae-abscessus infection (Fig. 2c).
Survival curves for nontuberculous mycobacterial lung disease (NTM-LD) with different radiographic patterns (cavitary vs. bronchiectatic vs. consolidative) were plotted by the Kaplan–Meier method and compared using the log-rank test in patients with a NTM-LD, b Mycobacterium avium complex, and c M. chelonae-abscessus. Black dots, patients still alive at the end of the study
The results of 6-month survival analysis of NTM-LD patients are summarized in Table 4. The model revealed that only consolidative radiographic pattern was an independent prognostic factor (HR: 4.55, 95% CI: 1.43–4.49), while history of prior pulmonary TB and comorbidity had borderline significance.
Discussion
NTM has become a more prevalent pathogen in pulmonary infection in recent decades [1, 20, 21]. The course of NTM-LD varies significantly, from chronic indolence to rapid progression [3, 6]. Consistent with previous reports [3, 11, 22], the current study shows that NTM-LD patients with different radiographic patterns have different clinical characteristics, laboratory findings, pulmonary function, and 6-month survival. Patients with cavitary NTM-LD are more likely to have a prior TB history, whereas those with consolidative pattern are more likely to have underlying comorbidity and poorer 6-month survival.
The pathogenesis of different radiographic patterns in NTM-LD is controversial. Host factors have been suggested because the virulence of MAC isolates from cavitary disease and nodular-bronchiectatic disease is similar in an in vitro study [23]. Structural lung disease is a well-documented risk factor of NTM-LD [6, 24]. Male predominance, presence of prior pulmonary TB, and poor baseline pulmonary function in patients with cavitary pattern suggest that prior pulmonary TB is the etiology of structural lung disease [25, 26]. In patients with bronchiectatic pattern, bronchiectasis itself may be the sole risk factor of NTM-LD [24, 27]. On the other hand, an immunocompromised state due to underlying comorbidity, rather than structural lung disease, seems to play an important role in developing a consolidative pattern.
The reasons why NTM-LD with radiographic consolidative pattern has the worst survival are probably multiple. First, these patients most likely have underlying comorbidities and poor nutritional status. Second, inflammation is most severe in consolidative NTM-LD. Third, only about 20% of consolidative NTM-LD patients receive antimycobacterial treatment because the consolidative pattern is not the usual radiographic manifestation of NTM-LD and its symptoms were frequently atypical. Clinicians treat it as bacterial pneumonia, and even if their sputum samples are smear-positive for AFB, antituberculous treatment is the usual prescription. The findings in the current study suggest that for patients with underlying comorbidities presenting with pulmonary consolidation, mycobacterial culture of sputum samples should be performed. Moreover, since prognosis is different in NTM-LD with different radiographic presentations, analysis of the outcome and treatment response of NTM-LD in clinical studies should be stratified by radiographic patterns.
In non-MAC-LD, the cavitary group has a 6-month survival rate similar to that of the bronchiectatic group, especially for those caused by M. chelonae-abscessus. This finding is different from a previous report on MAC-LD showing that the cavitary pattern has negative prognostic impact [11]. Differences in the survival impact of the cavitary pattern between MAC and non-MAC lung diseases suggest a different pathophysiology of pulmonary cavitation in different NTM species. This discrepancy is also probably due to the low virulence of most non-MAC species compared to MAC [3].
Fibrocavitary and nodular-bronchiectasis patterns used to be considered the most common radiographic findings of NTM-LD [3]. Recent studies reveal that bronchiectatic findings (62–66%) are more common than cavitation (26–47%), which is similar to the current results [9, 28, 29]. A possible explanation may be an underestimation of nodular-bronchiectasis by radiography in previous reports. With advances in mycobacterial culture, NTM are more frequently isolated from patients with nodular-bronchiectasis, which used to be considered as having lower mycobacterial load than cavitary lesions.
As regards the location of pulmonary involvement, the middle or lingular lung fields are the common sites infected by NTM, especially for the bronchiectasis type [2, 3, 28, 29]. A possible explanation for the upper-lobe predominance of bronchiectasis in the present study is the study area (Taiwan), which is still a TB endemic area and 19% of the bronchiectatic patients had prior history of TB. Post-tuberculosis bronchiectasis in the upper lungs is prone to NTM-LD [27].
Anti-NTM treatment is not associated with better survival in this study for various reasons. First, the timing of the start of antimycobacterial treatment and the regimens used are not standardized. About 40% of treated patients also received antituberculous treatment initially because of positive sputum smear for AFB [6, 30]. In this sense, the regimen contains no new macrolide and only rifampicin and ethambutol as possibly effective anti-NTM drugs for some slowly growing NTM but not for rapidly growing mycobacteria [3, 14]. Second, the follow-up duration may be too short to demonstrate the effect of treatment [3]. Lastly, about one-third of NTM patients have underlying comorbidities, which may alter the effect of treatment [4]. Prospective, long-term follow-up studies with standardized diagnostic tests and treatment regimens are needed.
This study has some limitations. First, the incidence of NTM-LD may have been underestimated because sputum mycobacterial studies are not routinely checked. In addition, the bronchiectatic group could be underestimated because a CT scan was not performed on every patient. Second, the study was conducted in a tertiary-care referral center and patients may have been more seriously ill, leading to a relatively high mortality rate. Furthermore, due to the small number of NTM-LD cases, definitive conclusions cannot be made.
In conclusion, consolidative NTM-LD patients usually have underlying comorbidities and the worst prognosis in all three radiographic patterns. Cavitary NTM-LD patients have male predominance and more prior TB history. In MAC-LD, patients with cavitary pattern have worse survival than those with bronchiectasis. Because varying radiographic patterns represent different prognoses, understanding patient characteristics of NTM-LD with different radiographic patterns is important.
References
Lai CC, Tan CK, Chou CH et al (2010) Increasing incidence of non-tuberculous mycobacteria, Taiwan, 2000–2008. Emerg Infect Dis 16(2):294–296
Field SK, Cowie RL (2006) Lung disease due to the more common non-tuberculous mycobacteria. Chest 129(6):1653–1672
Griffith DE, Aksamit T, Brown-Elliott BA et al (2007) An official ATS/IDSA statement: diagnosis, treatment, and prevention of non-tuberculous mycobacterial diseases. Am J Respir Crit Care Med 175(4):367–416
Chetchotisakd P, Kiertiburanakul S, Mootsikapun P, Assanasen S, Chaiwarith R, Anunnatsiri S (2007) Disseminated non-tuberculous mycobacterial infection in patients who are not infected with HIV in Thailand. Clin Infect Dis 45(4):421–427
Martin-Casabona N, Bahrmand AR, Bennedsen J et al (2004) Non-tuberculous mycobacteria: patterns of isolation. A multi-country retrospective survey. Int J Tuberc Lung Dis 8(10):1186–1193
Shu CC, Lee CH, Wang JY et al (2008) Nontuberculous mycobacteria pulmonary infection in medical intensive care unit: the incidence, patient characteristics, and clinical significance. Intensive Care Med 34(12):2194–2201
Donnabella V, Salazar-Schicchi J, Bonk S, Hanna B, Rom WN (2000) Increasing incidence of Mycobacterium xenopi at Bellevue Hospital: an emerging pathogen or a product of improved laboratory methods? Chest 118(5):1365–1370
Lu D, Heeren B, Dunne WM (2002) Comparison of the Automated Mycobacteria Growth Indicator Tube System (BACTEC 960/MGIT) with Lowenstein-Jensen medium for recovery of mycobacteria from clinical specimens. Am J Clin Pathol 118(4):542–545
Koh WJ, Yu CM, Suh GY et al (2006) Pulmonary TB and NTM lung disease: comparison of characteristics in patients with AFB smear-positive sputum. Int J Tuberc Lung Dis 10(9):1001–1007
Obayashi Y, Fujita J, Suemitsu I, Kamei T, Nii M, Takahara J (1998) Clinical features of non-tuberculous mycobacterial disease: comparisons between smear-positive and smear-negative cases, and between Mycobacterium avium and Mycobacterium intracellulare. Int J Tuberc Lung Dis 2(7):597–602
The Research Committee of the British Thoracic Society (2002) Pulmonary disease caused by Mycobacterium avium-intracellulare in HIV-negative patients: five-year follow-up of patients receiving standardised treatment. Int J Tuberc Lung Dis 6(7):628–634
Wang JY, Hsueh PR, Wang SK et al (2007) Disseminated tuberculosis: a 10-year experience in a medical center. Medicine 86(1):39–46
Pfyffer GE, Vicent V, Gutiérrez MC, Brown-Elliott BA, Wallace RJ (2007) Myobacterium. In: Murray PR, Baron EJ, Jorgensen JH, Pfaller MA, Landry ML (eds) Manual of clinical microbiology, 6th edn. American Society for Microbiology, Washington, DC, pp 543–600
American Thoracic Society, Centers for Disease Control and Prevention, Infectious Diseases Society of America (2005) American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: controlling tuberculosis in the United States. Am J Respir Crit Care Med 172(9):1169–1227
Ralph AP, Ardian M, Wiguna A et al (2010) A simple, valid, numerical score for grading chest x-ray severity in adult smear-positive pulmonary tuberculosis. Thorax 65(10):863–869
Iseman MD, Chan ED (2010) Bronchiectasis. In: Mason RJ, Broaddus VC, Martin TR, King TE, Schraufnagel DE, Murray JF, Nadel JA (eds) Murray and Nadel’s textbook of respiratory medicine, 5th edn. Elsevier, New York, pp 1023–1046
Godoy MC, Vos PM, Cooperberg PL, Lydell CP, Phillips P, Muller NL (2008) Chest radiographic and CT manifestations of chronic granulomatous disease in adults. Am J Roentgenol 191(5):1570–1575
Shu CC, Wu HD, Yu MC et al (2010) Use of high-dose inhaled corticosteroids is associated with pulmonary tuberculosis in patients with chronic obstructive pulmonary disease. Medicine (Baltimore) 89(1):53–61
Pellegrino R, Viegi G, Brusasco V et al (2005) Interpretative strategies for lung function tests. Eur Respir J 26(5):948–968
Kim RD, Greenberg DE, Ehrmantraut ME et al (2008) Pulmonary non-tuberculous mycobacterial disease: prospective study of a distinct pre-existing syndrome. Am J Respir Crit Care Med 178(10):1066–1074
Prevots DR, Shaw PA, Strickland D et al (2010) Non-tuberculous mycobacterial lung disease prevalence at four integrated healthcare delivery systems. Am J Respir Crit Care Med 182(7):970–976
Sohn H, Kim HJ, Kim JM, Jung Kwon O, Koh WJ, Shin SJ (2009) High virulent clinical isolates of Mycobacterium abscessus from patients with the upper lobe fibrocavitary form of pulmonary disease. Microb Pathog 47(6):321–328
Tatano Y, Yasumoto K, Shimizu T et al (2010) Comparative study for the virulence of Mycobacterium avium isolates from patients with nodular-bronchiectasis- and cavitary-type diseases. Eur J Clin Microbiol Infect Dis 29(7):801–806
Aksamit TR (2002) Mycobacterium avium complex pulmonary disease in patients with pre-existing lung disease. Clin Chest Med 23(3):643–653
Okumura M, Iwai K, Ogata H et al (2008) Clinical factors on cavitary and nodular bronchiectatic types in pulmonary Mycobacterium avium complex disease. Intern Med 47(16):1465–1472
Baig IM, Saeed W, Khalil KF (2010) Post-tuberculous chronic obstructive pulmonary disease. J Coll Physicians Surg Pak 20(8):542–544
Johnson MM, Waller EA, Leventhal JP (2008) Non-tuberculous mycobacterial pulmonary disease. Curr Opin Pulm Med 14(3):203–210
Koh WJ, Kwon OJ, Jeon K et al (2006) Clinical significance of non-tuberculous mycobacteria isolated from respiratory specimens in Korea. Chest 129(2):341–348
Marras TK, Mehta M, Chedore P, May K, Al Houqani M, Jamieson F (2010) Non-tuberculous mycobacterial lung infections in Ontario, Canada: clinical and microbiological characteristics. Lung 188(4):289–299
Shu CC, Lee LN, Wang JT, Chien YJ, Wang JY, Yu CJ (2010) Non-tuberculous mycobacterial pleurisy: an 8-year single-centre experience in Taiwan. Int J Tuberc Lung Dis 14(5):635–641
Acknowledgments
The authors thank the Institute for Biotechnology and Medicine Industry, Taiwan, for their academic funding and the National Taiwan University Hospital for funding (NTUH.100-N1685) and equipment support (the 8th Core Lab).
Disclosure
All of the authors declare that there were no financial, professional, or other personal interests of any nature related to a product, service, and/or company that would influence the study results.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Taiwan Anti-Mycobacteria Investigation (TAMI) group: Jann-Yuan Wang, Li-Na Lee, Chong-Jen Yu, Pan-Chyr Yang, Wei-Juin Su, Chin-Chung Shu, Hsin-Chih Lai, Chih-Hsin Lee, Ming-Chih Yu.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shu, CC., Lee, CH., Hsu, CL. et al. Clinical Characteristics and Prognosis of Nontuberculous Mycobacterial Lung Disease with Different Radiographic Patterns. Lung 189, 467–474 (2011). https://doi.org/10.1007/s00408-011-9321-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-011-9321-4