Abstract
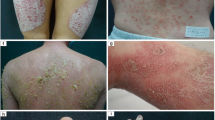
Psoriasis is an incurable cutaneous illness characterized by the presence of well-delimited reddish plaques and silvery-white dry scales. So far, there is a limited understanding of its pathogenesis, though recent discoveries on the immunological, genetic and molecular aspects of this disease have significantly contributed to the identification of new targets and the development of novel drugs. Despite these advances, many patients are still dissatisfied, so to improve patient satisfaction, reliability, and clinical outcomes, the individualization of the treatments for this disease becomes a necessity. This review summarizes recent findings related to psoriasis pathogenesis and describes new small molecules and targets recently identified as promising for treatments. Additionally, the current status, challenges and the future directions for achieving individualized therapy for this disease and the need for more collaborative studies are discussed. The individualization of treatments for psoriasis, rather than a goal, is analyzed as a process where a dynamic integration between the needs and characteristics of the patients, the pharmacological progress, and the clinical decisions takes place.




Similar content being viewed by others
References
Lowes MA, Su MIM, Loring B, John SM (2017) A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 31:205–212
Varma SR, Sivaprakasam TO, Mishra A, Prabhu S, Rafiq M, Rangesh P (2017) Imiquimod-induced psoriasis-like inflammation in differentiated Human keratinocytes: its evaluation using curcumin. Eur J Pharmacol. 813:33–41
Lowes MA, Suárez-Fariñas M, Krueger JG (2014) Immunology of psoriasis. Annu Rev Immunol 32:227–255
Callis Duffin K, Yeung H, Takeshita J, Krueger GG, Robertson AD, Troxel AB et al (2014) Patient satisfaction with treatments for moderate-to-severe plaque psoriasis in clinical practice. Br J Dermatol. 170:672–680
Schaarschmidt M-L, Kromer C, Herr R, Schmieder A, Goerdt S, Peitsch WK. Treatment satisfaction of patients with psoriasis [Internet]. 2015 [cited 2019 Jul 2]. https://www.ingentaconnect.com/content/mjl/adv/2015/00000095/00000005/art00011
Lesko LJ, Schmidt S (2012) Individualization of drug therapy: history, present state, and opportunities for the future. Clin Pharmacol Ther 92:458–466
van de Kerkhof PCM (2008) Options for the treatment of psoriasis: a multifactorial approach. Clin Dermatol 26:419–423
García-Pérez ME, Jean J, Pouliot R (2012) Antipsoriatic drug development: challenges and new emerging therapies. Recent Pat Inflamm Allergy Drug Discov 6:3–21
Lebwohl M (2016) Psoriasis therapy: breakthroughs in pharmacogenomics or in pharmacology? J Invest Dermatol 136:2339–2340
Woolf RT, Smith CH (2010) How genetic variation affects patient response and outcome to therapy for psoriasis. Expert Rev Clin Immunol 6:957–966
Ryan C, Menter A, Warren RB (2010) The latest advances in pharmacogenetics and pharmacogenomics in the treatment of psoriasis. Mol Diag Ther 14:81–93
O’Rielly DD, Rahman P (2010) Pharmacogenetics of psoriasis. Pharmacogenomics 12:87–101
Sutherland A, Power RJ, Rahman P, O’Rielly DD (2016) Pharmacogenetics and pharmacogenomics in psoriasis treatment: current challenges and future prospects. Expert Opin Drug Metab Toxicol 12:923–935
Ovejero-Benito MC, Muñoz-Aceituno E, Reolid A, Saiz-Rodríguez M, Abad-Santos F, Daudén E (2018) Pharmacogenetics and pharmacogenomics in moderate-to-severe psoriasis. Am J Clin Dermatol 19:209–222
Crowe JS, Roberts KJ, Carlton TM, Maggiore L, Cubitt MF, Clare S et al (2018) Preclinical development of a novel, orally-administered anti-tumour necrosis factor domain antibody for the treatment of inflammatory bowel disease. Sci Rep 8:4941
Bernard F-X, Morel F, Camus M, Pedretti N, Barrault C, Garnier J, et al. Keratinocytes under fire of proinflammatory cytokines: bona fide innate immune cells involved in the physiopathology of chronic atopic dermatitis and psoriasis [Internet]. J Allergy. 2012 [cited 2019 Jul 10]. https://www.hindawi.com/journals/ja/2012/718725/abs/
Chen L, Tsai T-F (2018) HLA-Cw6 and psoriasis. Br J Dermatol 178:854–862
Jabbari A, Johnson-Huang LM, Krueger JG (2011) Role of the immune system and immunological circuits in psoriasis. G Ital Dermatol Venereol 146:17–30
Cotsapas C, Voight BF, Rossin E, Lage K, Neale BM, Wallace C et al (2011) Pervasive sharing of genetic effects in autoimmune disease. PLoS Genet 7:e1002254
Tsoi LC, Spain SL, Knight J, Ellinghaus E, Stuart PE, Capon F et al (2012) Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nat Genet 44:1341–1348
Capon F (2017) The genetic basis of psoriasis. Int J Mol Sci [Internet]. [cited 2019 Jul 11];18. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5751129/
Hawkes JE, Chan TC, Krueger JG (2017) Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol 140:645–653
Lande R, Botti E, Jandus C, Dojcinovic D, Fanelli G, Conrad C et al (2014) The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat Commun 5:5621
Ganguly D, Chamilos G, Lande R, Gregorio J, Meller S, Facchinetti V et al (2009) Self-RNA–antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J Exp Med 206:1983–1994
Arakawa A, Siewert K, Stöhr J, Besgen P, Kim S-M, Rühl G et al (2015) Melanocyte antigen triggers autoimmunity in human psoriasis. J Exp Med 212:2203–2212
Bonifacio KM, Kunjravia N, Krueger JG, Fuentes-Duculan J (2016) Cutaneous expression of A disintegrin-like and metalloprotease domain containing thrombospondin type 1 motif-like 5 (ADAMTSL5) in psoriasis goes beyond melanocytes. J Pigment Disord [Internet]. [cited 2019 Jul 15];3. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5110039/
Cheung KL, Jarrett R, Subramaniam S, Salimi M, Gutowska-Owsiak D, Chen Y-L et al (2016) Psoriatic T cells recognize neolipid antigens generated by mast cell phospholipase delivered by exosomes and presented by CD1a. J Exp Med 213:2399–2412
The Immunologic Role of IL-17 in Psoriasis and Psoriatic Arthritis Pathogenesis—PubMed—NCBI [Internet]. [cited 2019 May 23]. https://www.ncbi.nlm.nih.gov/pubmed/30109481
Chiricozzi A, Guttman-Yassky E, Suárez-Fariñas M, Nograles KE, Tian S, Cardinale I et al (2011) Integrative responses to IL-17 and TNF-α in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J Invest Dermatol 131:677–687
van Baarsen LGM, Lebre MC, van der Coelen D, Aarrass S, Tang MW, Ramwadhdoebe TH et al (2014) Heterogeneous expression pattern of interleukin 17A (IL-17A), IL-17F and their receptors in synovium of rheumatoid arthritis, psoriatic arthritis and osteoarthritis: possible explanation for nonresponse to anti-IL-17 therapy? Arthritis Res Ther 16:426
Fan J, Lv Z, Yang G, Liao T ting, Xu J, Wu F et al. (2018) Retinoic acid receptor-related orphan receptors: critical roles in tumorigenesis. Front Immunol [Internet]. [cited 2019 Jul 31];9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5990620/
Peiser M (2013) Role of Th17 cells in skin inflammation of allergic contact dermatits. 2013:261037. https://doi.org/10.1155/2013/261037
Eberl G, Marmon S, Sunshine M, Rennert PD, Choi Y, Littman DR (2004) An essential function for the nuclear receptor ROR γ t in the generation of fetal lymphoid tissue inducer cells. Nat Immunol 5:64–73
Pandya VB, Kumar S, Sharma R, Desai RC. Combating Autoimmune Diseases With Retinoic Acid Receptor- Related Orphan Receptor- # ( ROR # or RORc ) Inhibitors : Hits and Misses. 2018;
Guntermann C, Piaia A, Hamel ML, Theil D, Rubic-Schneider T, del Rio-Espinola et al (2017) Retinoic-acid-orphan-receptor-C inhibition suppresses Th17 cells and induces thymic aberrations. JCI Insight. 2(5): e91127https://doi.org/10.1172/jci.insight.91127
Jetten AM (2009) Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. J Allergy 2012:718725 https://doi.org/10.1155/2012/718725
Fauber BP, Magnuson S (2014) Modulators of the nuclear receptor retinoic acid receptor-related orphan receptor- γ (ROR γ or RORc). J med chem 57:5871–5892
Turk DC, Okifuji A (1999) Assessment of patients’ reporting of pain : an integrated perspective. The Lancet 353:1784–1788
Driessen C, Bryant RAR, Villadangos JA, Bryant PW, Shi G, Chapman HA et al (1999) Cathepsin S controls the trafficking and maturation of MHC class II molecules in dendritic cells. J Cell biol 147:775–790
Petanceska S, Canoll P, Devi LA (1996) Expression of rat Cathepsin S in phagocytic cells. J Biol Chem 271:4403–4409
Scho A, Wendt W, Schattling B, Schulten R, Hoffmann K, Stuecker M et al (2009) Upregulation of cathepsin S in psoriatic keratinocytes. Exp Dermatol 19:8–10
Ainscough J, Macleod T, Mcgonagle D, Brakefield R, Baron JM, Alase A et al (2017) Cathepsin S is the major activator of the psoriasis-associated proinflammatory cytokine IL-36 γ. In: Proceedings of the national academy of sciences 114(3):201620954
Tortola L, Rosenwald E, Abel B, Blumberg H, Schäfer M, Coyle AJ et al (2012) Psoriasiform dermatitis is driven by IL-36—mediated DC-keratinocyte crosstalk. J Clin Invest 12(11):3955–3976
Schwarz G, Boehncke W, Braun M, Schro CJ, Burster T, Flad T et al (2002) Cathepsin S Activity is detectable in human keratinocytes and is selectively upregulated upon stimulation with interferon-gamma. J Invest Dermatol 119(1):44–49
Lacruz RS, Feske S (2015) Diseases caused by mutations in ORAI1 and STIM1. Ann N Y Acad Sci 1356:45–79
Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel S, Tanasa B et al (2006) A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441(7090):179–185
Putney JW (1991) Receptor-regulated calcium entry. Pharmacol Ther 48(3):427–434
Matsumoto M, Baby Y (2013) [Role of STIM-dependent Ca(2+) influx in regulatory b cells]. Yakugaku Zasshi 133(4):419–425
Bai S, Nagai M, Koerner SK, Veves A, Sun L (2016) Structure-activity relationship study and discovery of indazole 3- carboxamides as calcium-release activated calcium channel blockers. Bioorg Med Chem Lett 27(3):393–397
Steinckwich N, Myers P, Janardhan KS, Flagler ND, King D, Petranka JG et al (2015) Role of the store-operated calcium entry protein, STIM1, in neutrophil chemotaxis and infiltration into a murine model of psoriasis-in flamed skin. FASEB J 29(7):3003–3013
Karvonen SL, Korkiamäki T, Ylä-Outinen H, Nissinen M, Teerikangas H, Pummi K et al (2000) Psoriasis and altered calcium metabolism: downregulated capacitative calcium influx and defective calcium-mediated cell signaling in cultured psoriatic keratinocytes. J Invest Dermatol 114:693–700
Harteneck C, Friedland K (2014) Calcium—a central regulator of keratinocyte keratinocyte differentiation in health. Euro J Dermatol 24:650–661
Decherchi S, Berteotti A, Bottegoni G, Rocchia W, Cavalli A (2015) The ligand binding mechanism to purine nucleoside phosphorylase elucidated via molecular dynamics and machine learning. Nat Commun 6:1–10
Makita S, Maeshima AM, Maruyama D, Izutsu K, Tobinai K (2018) Forodesine in the treatment of relapsed/refractory peripheral T-cell lymphoma: an evidence-based review. Onco Targets Therapy 11:2287–2293
Balakrishnan K, Nimmanapalli R, Ravand F, Keating MJ, Ghandi V (2006) Forodesine, an inhibitor of purine nucleoside phosphorylase, induces apoptosis in chronic lymphocytic leukemia cells. Blood 108:2392–2398
Bantia S, Parker C, Upshaw R, Cunningham A, Kotian P, Kilpatrick JM et al (2010) Potent orally bioavailable purine nucleoside phosphorylase inhibitor BCX-4208 induces apoptosis in B- and T-lymphocytes—a novel treatment approach for autoimmune diseases, organ transplantation and hematologic malignancies. Int Immunopharmacol 10:784–790
Kumar N, Goldminz AM, Kim N, Gottlieb AB (2013) Phosphodiesterase 4-targeted treatments for autoimmune diseases. BMC Med 11:1–8
Lin C-H, Chang S-H, Fang J-Y (2016) Recent advances using phosphodiesterase 4 (PDE4) inhibitors to treat inflammatory disorders: animal and clinical studies. Curr Drug Therapy 11:21–40
Raker VK, Becker C, Steinbrink K. The cAMP Pathway as therapeutic target in autoimmune and inflammatory diseases. Front Immunol [Internet]. 2016 [cited 2018 Nov 19];7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4814577/
Papp K, Reich K, Leonardi CL, Kircik L, Chimenti S, Langley RGB et al (2015) Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (efficacy and safety trial evaluating the effects of apremilast in psoriasis [ESTEEM] 1). J Am Acad Dermatol 73:37–49
Lim CP, Xinmin C (2006) Structure, function, and regulation of STAT proteins. R Soc Chem. 2:536–550
Harden JL, Krueger JG, Bowcock AM (2015) The immunogenetics of psoriasis: a comprehensive review. J Autoimmun Elsevier Ltd 64:66–73
Ibrahim DA, Khattab FM (2016) Signal transducer and activator of transcription 3 and vascular endothelial growth factor expression in psoriasis, an immunohistochemical study. Egypt J Pathol 36:229–234
Hsu L, Armstrong AW (2014) JAK inhibitors: Treatment efficacy and safety profile in patients with psoriasis. J Immunol Res 2014(3):283617
Rawlings JS (2004) The JAK/STAT signaling pathway. J Cell Sci 117:1281–1283
Andrés RM, Hald A, Johansen C, Kragballe K, Iversen L (2013) Studies of Jak/STAT3 expression and signalling in psoriasis identifies STAT3-Ser727 phosphorylation as a modulator of transcriptional activity. Exp Dermatol 22:323–328
Calautti E, Avalle L, Poli V (2018) Psoriasis: A STAT3-centric view. Int J Mol Sci 19:171
Tohyama M, Shirakata Y, Hanakawa Y, Dai X, Shiraishi K, Murakami M et al (2018) Bcl-3 induced by IL-22 via STAT3 activation acts as a potentiator of psoriasis-related gene expression in epidermal keratinocytes. Eur J Immunol 48:168–179
Wu P, Ma G, Zhu X, Gu T, Zhang J, Sun Y et al (2017) Cyr61/CCN1 is involved in the pathogenesis of psoriasis vulgaris via promoting IL-8 production by keratinocytes in a JNK/NF-κB pathway. Clin Immunol 174:53–62 (Elsevier Inc.)
Gambichler T, Skrygan M (2015) Expression of human β-defensin-2 in psoriatic epidermis models treated with balneophototherapy. J Eur Acad Dermatol Venereol 29:169–173
Hawkes JE, Chan TC, Krueger JG (2017) Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol. 140:645–653 (Elsevier Inc.)
Yang L, Jin L, Ke Y, Fan X, Zhang T, Zhang C et al (2018) E3 ligase Trim21 ubiquitylates and stabilizes keratin 17 to induce STAT3 activation in psoriasis. J Investig Dermatol Authors 32:1–10
Alao JP (2007) The regulation of cyclin D1 degradation: Roles in cancer development and the potential for therapeutic invention. Mol Cancer 6:1–16
Yiu ZZN, Warren RB (2016) Novel oral therapies for psoriasis and psoriatic arthritis. Am J Clin Dermatol 17:191–200
Su Y, Wang Q, Yang B, Wu L, Cheng G, Kuang H (2017) Withasteroid B from D. metel L. regulates immune responses by modulating the JAK/STAT pathway and the IL-17+ RORγt+ /IL-10+ FoxP3+ ratio. Clin Exp Immunol 190:40–53
Nadeem A, Al-Harbi NO, Al-Harbi MM, El-Sherbeeny AM, Ahmad SF, Siddiqui N et al (2015) Imiquimod-induced psoriasis-like skin inflammation is suppressed by BET bromodomain inhibitor in mice through RORC/IL-17A pathway modulation. Pharmacolo res 99:248–257
Fauber BP, René O, Deng Y, DeVoss J, Eidenschenk C, Everett C et al (2015) Discovery of 1-{4-[3-fluoro-4-((3s,6r)-3-methyl-1,1-dioxo-6-phenyl-[1,2]thiazinan-2-ylmethyl)-phenyl]-piperazin-1-yl}-ethanone (GNE-3500): a potent, selective, and orally bioavailable retinoic acid receptor-related orphan receptor C (RORc or RORγ) inverse agonist. J Med Chem 58:5308–5322
A Study of GSK2981278 Ointment in subjects with plaque psoriasis—study results—ClinicalTrials.gov [Internet]. [cited 2019 Aug 1]. https://clinicaltrials.gov/ct2/show/results/NCT03004846
Ouvry G, Atrux-Tallau N, Bihl F, Bondu A, Bouix-Peter C, Carlavan I et al (2018) Discovery and characterization of CD12681, a potent RORγ inverse agonist, preclinical candidate for the topical treatment of psoriasis. Chem Med Chem 13:321–337
Gege C (2017) RORγt inhibitors as potential back-ups for the phase II candidate VTP-43742 from Vitae Pharmaceuticals: Patent Evaluation of WO2016061160 and US20160122345. Expert Opin Ther Pat 27:1–8
Skurkovich SV, Skurkovich B, Kelly JA (2002) Anticytokine therapy—new approach to the treatment of autoimmune and cytokine-disturbance diseases. Med hypotheses 59:770–780
Muqit MMK, Abou-sleiman PM, Saurin AT, Harvey K, Deas E, Eaton S et al (2006) Altered cleavage and localization of PINK1 to aggresomes in the presence of proteasomal stress. J Neurochem 98(1):156–169
Markt P, Mcgoohan C, Walker B, Kirchmair J, Feldmann C, Martino De G et al (2008) Discovery of novel Cathepsin S inhibitors by pharmacophore-based virtual high-throughput screening. J Chem Inf Model 48(8):1693–1705. https://doi.org/10.1021/ci80010j
Gauthier JY, Black WC, Courchesne I, Cromlish W, Desmarais S, Houle R et al (2007) The identification of potent, selective, and bioavailable cathepsin S inhibitors. Bioorg Med Chem Lett 17(17):4929–4933
Lee-Dutra A, Wiener DK, Sun S (2011) Cathepsin S inhibitors: 2004–2010. Expert Opin Ther Pat 21:311–337
Baugh M, Black D, Westwood P, Kinghorn E, Mcgregor K, Bruin J et al (2015) Therapeutic dosing of an orally active, selective cathepsin S inhibitor suppresses disease in models of autoimmunity. J Autoimmun Elsevier Ltd 36:201–209
Liu W, Hickey ER. Chapter 11—Protease inhibitors for the potential treatment of chronic obstructive pulmonary disease and asthma. In: Macor JE (ed) Annual reports in medicinal chemistry [Internet]. Academic Press; 2008 [cited 2019 Aug 2]. pp 171–85. https://www.sciencedirect.com/science/article/pii/S0065774308000110
Schering AG. Celera Genomics announces the sale of its cathepsin S inhibitor program to Schering AG. www.celera.com/celera/pr_1152570053. 21 June 2006.
Alza Corporation. Study to investigate the safety, tolerability, absorption, distribution, metabolism, and elimination of RWJ-445380 administered to patients with plaque psoriasis—NCT00396422 [Internet]. ClinicalTrials.gov. [cited 2016 Nov 12]. https://clinicaltrials.gov/ct2/show/NCT00396422?term=RWJ-445380&rank=1
Tian C, Du L, Zhou Y, Li M (2016) Store-operated CRAC channel inhibitors: opportunities and challenges. Future Med Chem 8:817–832
G Velicelebi, K Stauderman, J Whitten, Y Pei, J Cao, Z Wang, E Rogers, B Dyck, J Grey. Substituted thiophene modulators of intracellular calcium.
Roche and Biocryst Pharmaceuticals Advance BCX-4208/R3421 Into Phase II Psoriasis Trial [Internet]. BioSpace. 2007 [cited 2019 Jan 22]. https://www.biospace.com/article/roche-and-biocryst-pharmaceuticals-advance-bcx-4208-r3421-into-phase-ii-psoriasis-trial
Al-Kali A, Gandhi V, Ayoubi M, Keating M, Ravandi F (2010) Forodesine: review of preclinical and clinical data. Future Oncol 6:1211–1217
Shih H-P, Zhang X, Aronov AM (2017) Drug discovery effectiveness from the standpoint of therapeutic mechanisms and indications. Nat Rev Drug Discovery 17:19–33
Qing-Hui W, Lang-Hong W, Xin-An Z, De-Bao N, Man-Sheng W (2018) Hydroxyl-related differences for three dietary flavonoids as inhibitors of human purine nucleoside phosphorylase. Int J Biol Macromol 118:588–598
Abdulrahim H, Thistleton S, Adebajo AO, Shaw T, Edwards C, Wells A (2015) Apremilast: a PDE4 inhibitor for the treatment of psoriatic arthritis. Expert Opin Pharmacother 16:1099–1108
OTEZLA (apremilast) for Plaque Psoriasis and Psoriatic Arthritis [Internet]. Otezla Global. [cited 2019 Jan 22]. https://www.otezla.net/
Paul C, Cather J, Gooderham M, Poulin Y, Mrowietz U, Ferrandiz C et al (2015) Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol 173:1387–1399
Immune Metabolic Associations in Psoriatic Arthritis [Internet]. ClinicalTrials.gov. [cited 2019 Jan 22]. https://clinicaltrials.gov/ct2/show/NCT03399708
Stein Gold L, Bagel J, Lebwohl M, Jackson JM, Chen R, Goncalves J et al (2018) Efficacy and safety of apremilast in systemic- and biologic-naive patients with moderate plaque psoriasis: 52-week results of UNVEIL. J Drugs Dermatol 17:221–228
Multiple Ascending Dose Study to assess safety and pharmacokinetics of Hemay005 in healthy subjects [Internet]. ClinicalTrials.gov. [cited 2019 Jan 22]. https://clinicaltrials.gov/ct2/show/NCT03570346
Liu X, Chen R, Zeng G, Gao Y, Liu X, Zhang D et al (2018) Determination of a PDE4 inhibitor Hemay005 in human plasma and urine by UPLC–MS/MS and its application to a PK study. Bioanalysis 10:863–875
AN2728 Topical Ointment to Treat Mild-to-Moderate Plaque-Type Psoriasis [Internet]. ClinicalTrials.gov. [cited 2019 Jan 22]. https://clinicaltrials.gov/ct2/show/NCT01300052
Lee EB, Lebwohl MG, Wu JJ (2019) Treatment of psoriasis with crisaborole. J Dermatol Treat 30(2):156–157
Sharma M, Levenson C, Clements I, Castella P, Gebauer K, Cox ME. East Indian Sandalwood Oil (EISO) Alleviates inflammatory and proliferative pathologies of psoriasis. Front Pharmacol [Internet]. 2017 [cited 2018 Dec 1];8. https://journal.frontiersin.org/article/10.3389/fphar.2017.00125/full
A Trial of a Botanical Drug (EISO) for treatment of mild-to-moderate plaque psoriasis [Internet]. ClinicalTrials.gov. [cited 2019 Jan 22]. https://clinicaltrials.gov/ct2/show/NCT03000608
Papp K, Pariser D, Catlin M, Wierz G, Ball G, Akinlade B et al (2015) A phase 2a randomized, double-blind, placebo-controlled, sequential dose-escalation study to evaluate the efficacy and safety of ASP015K, a novel Janus kinase inhibitor, in patients with moderate-to-severe psoriasis. Br J Dermatol 173:767–776
Papp KA, Menter MA, Raman M, Disch D, Schlichting DE, Gaich C et al (2016) A randomized phase 2b trial of baricitinib, an oral Janus kinase (JAK) 1/JAK2 inhibitor, in patients with moderate-to-severe psoriasis. Br J Dermatol 174:1266–1276
A Study of Escalating Doses of Itacitinib Administered Orally in Patients With Plaque Psoriasis—Full Text View—ClinicalTrials.gov [Internet]. [cited 2019 Aug 2]. https://clinicaltrials.gov/ct2/show/NCT01634087
Bissonnette R, Luchi M, Fidelus-Gort R, Jackson S, Zhang H, Flores R et al (2016) A randomized, double-blind, placebo-controlled, dose-escalation study of the safety and efficacy of INCB039110, an oral janus kinase 1 inhibitor, in patients with stable, chronic plaque psoriasis. J Dermatol Treat 27:332–338
Miyoshi K, Takaishi M, Nakajima K, Ikeda M, Kanda T, Tarutani M et al (2011) Stat3 as a therapeutic target for the treatment of psoriasis: a clinical feasibility study with STA-21, a Stat3 Inhibitor. J Investig Dermatol Nat Publ Gr 131:108–117
Li K, Huang CC, Randazzo B, Li S, Szapary P, Curran M et al (2016) HLA-C*06:02 allele and response to IL-12/23 inhibition: results from the ustekinumab phase 3 psoriasis program. J Invest Dermatol 136:2364–2371
Grozdev I, Korman N, Tsankov N (2014) Psoriasis as a systemic disease. Clin Dermatol 32:343–350
Machado-Pinto J, Diniz MDS, Bavoso NC, Machado-Pinto J, Diniz MDS, Bavoso NC (2016) Psoriasis: new comorbidities. Anais Brasileiros de Dermatol. 91:8–14
Torres T, Romanelli M, Chiricozzi A (2016) A revolutionary therapeutic approach for psoriasis: bispecific biological agents. Expert Opin Investig Drugs 25:751–754
Goldenberg G, Lanoue J, Dong J (2016) New oral therapies for psoriasis. J Clin Aesthet Dermatol 9:25–28
Hermans C, Herranz P, Segaert S, Gils A (2017) Current practice of therapeutic drug monitoring of biopharmaceuticals in psoriasis patients. Ther Drug Monit 39:356
Papp KA, Langley RG, Lebwohl M, Krueger GG, Szapary P, Yeilding N et al (2008) Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet 371:1675–1684
Lavori PW, Dawson R (2008) Adaptive treatment strategies in chronic disease. Annu Rev Med 59:443–453
Mrowietz U, Kragballe K, Nast A, Reich K (2011) Strategies for improving the quality of care in psoriasis with the use of treatment goals—a report on an implementation meeting. J Eur Acad Dermatol Venereol 25:1–13
Strober BE, van der Walt JM, Armstrong AW, Bourcier M, Carvalho AVE, Chouela E et al (2019) Clinical goals and barriers to effective psoriasis care. Dermatol Ther (Heidelb) 9:5–18
Kitchen H, Cordingley L, Young H, Griffiths CEM, Bundy C (2015) Patient-reported outcome measures in psoriasis: the good, the bad and the missing! Br J Dermatol 172:1210–1221
Langley RG, Tsai T-F, Flavin S, Song M, Randazzo B, Wasfi Y et al (2018) Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol 178:114–123
Acknowledgements
The authors are very grateful to Roberto Esquivel-García for the technical support done in the scientific illustrations.
Funding
This article has no funding source.
Author information
Authors and Affiliations
Contributions
MEGP had the idea to write this review. All authors contributed to the design, literature searching, writing, and editing. MCBC, ARRO, and MEGP critically revised the work at multiple timepoints.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest, financial, or otherwise.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Claudia, CD., María-Elena, VH., Josué, VE. et al. Small molecules under development for psoriasis: on the road to the individualized therapies. Arch Dermatol Res 312, 611–627 (2020). https://doi.org/10.1007/s00403-020-02056-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-020-02056-3