Abstract
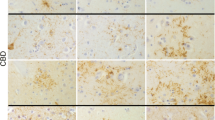
We investigated the occurrence and distribution of tuft-shaped astrocytes (TuSAs) in 60 brains from patients with Lewy body disease (LBD), which were clinically diagnosed as Parkinson’s disease (PD) or dementia with Lewy bodies (DLB), and 85 brains from control subjects. TuSAs have been documented as a neuropathological hallmark of progressive supranuclear palsy (PSP). We found phosphorylated tau (p-tau)-positive and α-synuclein-negative TuSAs in 10 of 60 patients with LBD and 3 of 85 control cases. TuSAs were mainly located within the precentral and premotor gyri of the frontal lobe cortex. There were only few TuSAs, but the distribution pattern and morphological and immunohistological features were similar to that in PSP. Furthermore, other p-tau positive structures, including aggregates in neurons, coiled-like glial cells and threads showed a similar distribution to those in PSP; mainly in the hippocampus, striatum, subthalamic nucleus, precentral and premotor gyri, brainstem nucleus, and dentate nucleus. In these cases, however, neuronal loss and gliosis were not seen in the regions involved in PSP, such as the subthalamic nucleus, pallidum, inferior olivary, cerebellar dentate nuclei, and periaqueductal gray matter. Clinical features were indistinguishable between the LBD with and without TuSAs. The appearance of TuSAs was not related to the frequency of Lewy bodies, neurofibrillary tangles, and senile plaques, but was significantly more pronounced with advancing age in both LBD and controls. These findings suggest that in a subgroup of elderly individual cases, especially associated with LB pathology, the glial and neuronal p-tau accumulation is increased and has a distributional pattern similar to PSP.



Similar content being viewed by others
References
Arai T, Ueda K, Ikeda K, Akiyama H, Haga C, Kondo H, Kuroki N, Niizato K, Iritani S, Tsuchiya K (1999) Argyrophilic glial inclusions in the midbrain of patients with Parkinson’s disease and diffuse Lewy body disease are immunopositive for NACP/α-synuclein. Neurosci Lett 259:83–86
Beach TG, Sue L, Scott S, Layne K, Newell A, Walker D, Baker M, Sahara N, Yen SH, Hutton M, Caselli R, Adler C, Connor D, Sabbagh M (2003) Hippocampal sclerosis dementia with tauopathy. Brain Pathol 13:263–278
Bergeron C, Pollanen MS, Weyer L, Lang AE (1997) Cortical degeneration in progressive supranuclear palsy. A comparison with cortical-basal ganglionic degeneration. J Neuropathol Exp Neurol 56:726–734
Braak E, Braak H (1991) Neuropathological staging of Alzheimer-related changes. Acta Neuropathol 82:239–259
Braak H, Braak E (1989) Cortical and subcortical argyrophilic grains characterize a disease associated with adult onset dementia. Neuropathol Appl Neurobiol 15:13–26
Chin SS, Goldman JE (1996) Glial inclusions in CNS degenerative diseases. J Neuropathol Exp Neurol 55:499–508
Dickson DW (1999) Neuropathologic differentiation of progressive supranuclear palsy and corticobasal degeneration. J Neurol 246 (Suppl 2):II6–15
Feany MB, Dickson DW (1995) Comparison of neurofibrillary lesions in progressive supranuclear palsy, Pick’s disease and corticobasal degeneration. J Neuropathol Exp Neurol 54:447
Feany MB, Dickson DW (1995) Widespread cytoskeletal pathology characterizes corticobasal degeneration. Am J Pathol 146:1388–1396
Feany MB, Mattiace LA, Dickson DW (1996) Neuropathologic overlap of progressive supranuclear palsy, Pick’s disease and corticobasal degeneration. J Neuropathol Exp Neurol 55:53–67
Gearing M, Olson DA, Watts RL, Mirra SS (1994) Progressive supranuclear palsy: neuropathologic and clinical heterogeneity. Neurology 44:1015–1024
Goedert M, Spillantini MG, Cairns NJ, Crowther RA (1992) Tau proteins of Alzheimer paired helical filaments: abnormal phosphorylation of all six brain isoforms. Neuron 8:159–168
Gomori AJ, Sima AA (1984) An autopsy case of progressive supranuclear palsy. Can J Neurol Sci 11:48–52
Hanihara T, Amano N, Takahashi H, Nagotomo H, Yagishita S (1995) Distribution of tangles and threads in the cerebral cortex in progressive supranuclear palsy. Neuropathol Appl Neurobiol 21:319–326
Haraguchi T, Ishizu H, Terada S, Takehisa Y, Tanabe Y, Nishinaka T, Kawai K, Kuroda S, Komoto Y, Namba M (2000) An autopsy case of postencephalitic parkinsonism of von Economo type: some new observations concerning neurofibrillary tangles and astrocytic tangles. Neuropathology 20:143–148
Hashimoto N, Takeuchi T, Ishihara R, Ukai K, Kobayashi H, Iwata H, Iwai K, Mizuno Y, Yamaguchi H, Shibayama H (2003) Glial fibrillary tangles in diffuse neurofibrillary tangles with calcification. Acta Neuropathol 106:150–156
Hauw JJ, Daniel SE, Dickson D, Horoupian DS, Jellinger K, Lantos PL, McKee A, Tabaton M, Litvan I (1994) Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology 44:2015–2019
Hishikawa N, HashizumeY, Yoshida M, Sobue G (2001) Widespread occurrence of argyrophilic glial inclusions in Parkinson’s disease. Neuropathol Appl Neurobiol 27:362–372
Hishikawa N, HashizumeY, Yoshida M, Sobue G (2003) Clinical neuropathological correlates of Lewy body disease. Acta Neuropathol 105:341–350
Hishikawa N, Niwa J, Doyu M, Ito T, Ishigaki S, Hashizume Y, Sobue G (2003) Dorfin localized to the ubiquitylated inclusions in Parkinson’s disease, dementia with Lewy bodies, multiple system atrophy, and amyotrophic lateral sclerosis. Am J Pathol 163:609–619
Ikeda K, Akiyama H, Kondo H, Ikeda K (1993) Anti-tau-positive glial fibrillary tangles in the brain of postencephalitic parkinsonism of Economo type. Neurosci Lett 162:176–178
Ikeda K, Akiyama H, Arai T, Nishimura T (1998) Glial tau pathology in neurodegenerative diseases: their nature and comparison with neuronal tangles. Neurobiol Aging 19:85–91
Iseki E, Togo T, Suzuki K, Katsuse O, Marui W, Silva de R, Lees A, Yamamoto T, Kosaka K (2003) Dementia with Lewy bodies from the perspective of tauopathy. Acta Neuropathol 105:265–270
Ishizu H, Kuroda S, Nishinaka T, SatoY, Namba M (1995) Glail tangles in Pick’s disease. Neuropathology 15:163–174
Iwatsubo T, Hasegawa M, Ihara Y (1994) Neuronal and glial tau-positive inclusions in diverse neurologic disease share common phosphorylation characteristics. Acta Neuropathol 88:129–136
Jellinger K, Riederer P, Tomonaga M (1980) Progressive supranuclear palsy: clinico-pathological and biochemical studies. J Neural Transm (Suppl) 16:111–128
Komori T, Arai N, Oda M, Nakayama H, Mori H, Yagishita S, Takahashi T, Amano N, Murayama S, Murakami S, Shibata N, Kobayashi M, Sasaki S, Iwata M (1998) Astrocytic plaques and tufts of abnormal fibers do not coexist in corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol 96:401–408
Matsusaka H, Ikeda K, Akiyama H, Arai T, Inoue M, Yagishita S (1998) Astrocytic pathology in progressive supranuclear palsy: significance for neuropathological diagnosis. Acta Neuropathol 96:248–252
Matsushita M, Ito K, Oyanagi S, Uchikoshi T, Ishiko T, Kase M, Kosaka K (1980) An autopsy case of progressive supranuclear palsy with massive appearance of neurofibrillary tangles in the limbic system including nucl. accumbens septi and nucl. amygdala. Neuropathology 1:119–132
McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne EJ, Lennox G, Quinn NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller BL, Lovestone S, Collerton D, Jansen ENH, Ballard C, Vos RA de, Wilcock GK, Jellinger KA, Perry RH (1996) Consensus guidelines for the clinical and pathological diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology 47:1113–1124
Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, Belle G van, Berg L (1991) The consortium to establish a registry for Alzheimer’s disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41:479–486
Mori H, Yoshimura M, Tomonaga M, Yamanouchi H (1986) Progressive supranuclear palsy with Lewy bodies. Acta Neuropathol 71:344–346
Mori H, Oda M, Komori T, Arai N, Takanashi M, Mizutani T, Hirai S, Mizuno Y (2002) Lewy bodies in progressive supranuclear palsy. Acta Neuropathol 104:273–278
Nishimura M, Tomimoto H, Suenaga T, Namba Y, Ikeda K, Akiguchi I, Kimura J (1995) Immunocytochemical characterization of glial fibrillary tangles in Alzheimer’s disease brain. Am J Pathol 146:1052–1058
Sergeant N, Wattez A, Delacourte A (1999) Neurofibrillary degeneration in progressive supranuclear palsy and corticobasal degeneration: tau pathologies with exclusively “exon 10” isoforms. J Neurochem 72:1243–1249
Spillantini MG, Goedert M (1998) Tau protein pathology in neurodegenerative diseases. Trends Neurosci 21:428–433
Takeda A, Mallory M, Sundsmo M, Honer W, Hansen L, Masliah E (1998) Abnormal accumulation of NACP/α-synuclein in neurodegenerative disorders. Am J Pathol 152:367–372
Tsuboi Y, Ahlskog JE, Apaydin H, Parisi JE, Dickson DW (2001) Lewy bodies are not increased in progressive supranuclear palsy compared with normal controls. Neurology 57:1675–1678
Wakabayashi K, Takahashi H (1996) Gallyas-positive, tau-negative glial inclusions in Parkinson’s disease midbrain. Neurosci Lett 217:133–136
Wakabayashi K, Oyanagi K, Makifuchi T, Ikuta F, Homma A, Homma Y, Horikawa Y, Tokiguchi S (1994) Corticobasal degeneration: etiopathological significance of the cytoskeletal alterations. Acta Neuropathol 87:545–533
Wakabayashi K, Hayashi S, Kakita A, Yamada M, Toyoshima Y, Yoshimoto M, Takahashi H (1998) Accumulation of α-synuclein/NACP is a cytopathological feature common to Lewy body disease and multiple system atrophy. Acta Neuropathol 96:445–452
Wakabayashi K, Hayashi S, Yoshimoto M, Kudo H, Takahashi H (2000) NACP/α-synuclein-positive filamentous inclusions in astrocytes and oligodendrocytes of Parkinson’s disease brains. Acta Neuropathol 99:14–20
Yang W, Sopper MM, Leystra-Lantz C, Strong MJ (2003) Microtubule-associated tau protein positive neuronal and glial inclusions in ALS. Neurology 61:1766–1773
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hishikawa, N., Hashizume, Y., Yoshida, M. et al. Tuft-shaped astrocytes in Lewy body disease. Acta Neuropathol 109, 373–380 (2005). https://doi.org/10.1007/s00401-004-0967-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-004-0967-3