Abstract
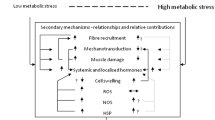
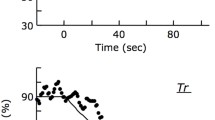
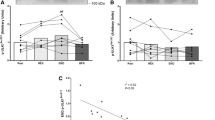
Skeletal muscle is a plastic organ that adapts its mass to various stresses by affecting pathways that regulate protein synthesis and degradation. This study investigated the effects of repetitive restriction of muscle blood flow (RRMBF) on microvascular oxygen pressure (PmvO2), mammalian target of rapamycin (mTOR) signaling pathways, and transcripts associated with proteolysis in rat skeletal muscle. Eleven-week-old male Wistar rats under anesthesia underwent six RRMBF consisting of an external compressive force of 100 mmHg for 5 min applied to the proximal portion of the right thigh, each followed by 3 min rest. During RRMBF, PmvO2 was measured by phosphorescence quenching techniques. The total RNA and protein of the tibialis anterior muscle were obtained from control rats, and rats treated with RRMBF 0–6 h after the stimuli. The protein expression and phosphorylation of various signaling proteins were determined by western blotting. The mRNA expression level was measured by real-time RT-PCR analysis. The total muscle weight increased in rats 0 h after RRMBF, but not in rats 1–6 h. During RRMBF, PmvO2 significantly decreased (36.1 ± 5.7 to 5.9 ± 1.7 torr), and recovered at rest period. RRMBF significantly increased phosphorylation of p70 S6-kinase (p70S6k), a downstream target of mTOR, and ribosomal protein S6 1 h after the stimuli. The protein level of REDD1 and phosphorylation of AMPK and MAPKs did not change. The mRNA expression levels of FOXO3a, MuRF-1, and myostatin were not significantly altered. These results suggested that RRMBF significantly decreased PmvO2, and enhanced mTOR signaling pathways in skeletal muscle using a rat model, which may play a role in diminishing muscle atrophy under various conditions in human studies.







Similar content being viewed by others
Abbreviations
- RMBF:
-
Restriction of muscle blood flow
- RRMBF:
-
Repetitive restriction of muscle blood flow
- PmvO2 :
-
Microvascular oxygen pressure
- HIF-1α:
-
Hypoxia-inducible factor-1α
- mTOR:
-
Mammalian target of rapamycin
- p70S6k:
-
p70 S6-kinase
- ERK1/2:
-
Extracellular signal-regulated kinase 1/2
- FOXO3a:
-
Forkhead box O3A
- MuRF-1:
-
Muscle ring finger-1
- VEGF:
-
Vascular endothelial growth factor
References
Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL (2004) Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117:399–412
Lang T, Streeper T, Cawthon P, Baldwin K, Taaffe DR, Harris TB (2010) Sarcopenia: etiology, clinical consequences, intervention, and assessment. Osteoporos Int 21:543–559
Koopman R, Zorenc AHG, Gransier RJJ, Cameron-Smith D, van Loon LJC (2006) Increase in S6K1 phosphorylation in human skeletal muscle following resistance exercise occurs mainly in type II muscle fibers. Am J Physiol Endocrinol Metab 290:E1245–E1252
Wilkinson SB, Phillips SM, Atherton PJ, Patel R, Yarasheski KE, Tarnopolsky MA, Rennie MJ (2008) Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol 586(Pt 15):3701–3717
Takano H, Morita T, Iida H, Asada K, Kato M, Uno K, Hirose K, Matsumoto A, Takenaka K, Hirata Y, Eto F, Nagai R, Sato Y, Nakajima T (2005) Hemodynamic and hormonal responses to a short-term low-intensity resistance exercise with the reduction of muscle blood flow. Eur J Appl Physiol 95:65–73
Abe T, Kearns CF, Sato Y (2006) Muscle size and strength are increased following walk training with restricted venous blood flow from the leg muscle, Kaatsu-walk training. J Appl Physiol 100:1460–1466
Takarada Y, Takazawa H, Sato Y, Takebayashi S, Tanaka Y, Ishii N (2000) Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J Appl Physiol 88:2097–2106
Fujita S, Abe T, Drummond MJ, Cadenas JG, Dreyer HC, Sato Y, Volpi E, Rasmussen BB (2007) Blood flow restriction during low-intensity resistance exercise increases S6K1 phosphorylation and muscle protein synthesis. J Appl Physiol 103:903–910
Manini TM, Vincent KR, Leeuwenburgh CL, Lees HA, Kavazis AN, Borst SE, Clark BC (2011) Myogenic and proteolytic mRNA expression following blood flow restricted exercise. Acta Physiol (Oxf) 201:255–263
Laurentino GC, Ugrinowitsc C, Roschel H, Aoki MS, Soares AG, Neves M Jr, Aihara AY, Fernandes Ada R, Tricoli V (2012) Strength training with blood flow restriction diminishes myostatin gene expression. Med Sci Sports Exerc 44:406–412
Takarada Y, Takazawa H, Ishii N (2000) Applications of vascular occlusion diminish disuse atrophy of knee extensor muscles. Med Sci Sports Exerc 32:2035–2039
Kubota A, Sakuraba K, Sawaki K, Sumide T, Tamura Y (2008) Prevention of disuse muscular weakness by restriction of blood flow. Med Sci Sports Exerc 40:529–534
Kano Y, Masuda K, Furukawa H, Sudo M, Mito K, Sakamoto K (2008) Histological skeletal muscle damage and surface EMG relationships following eccentric contractions. J Physiol Sci 58:349–355
Koga S, Kano Y, Barstow TJ, Ferreira LF, Ohmae E, Sudo M, Poole DC (2012) Kinetics of muscle deoxygenation and microvascular PO(2) during contractions in rat: comparison of optical spectroscopy and phosphorescence-quenching techniques. J Appl Physiol 112:26–32
Poole DC, Wagner PD, Wilson DF (1995) Diaphragm microvascular plasma PO2 measured in vivo. J Appl Physiol 79:2050–2057
Rumsey WL, Vanderkooi JM, Wilson DF (1988) Imaging of phosphorescence: a novel method for measuring oxygen distribution in perfused tissue. Science 241:1649–1651
Lo LW, Vinogradov SA, Koch CJ, Wilson DF (1997) A new, water soluble, phosphor for oxygen measurements in vivo. Adv Exp Med Biol 428:651–656
Poole DC, Behnke BJ, McDonough P, McAllister RM, Wilson DF (2004) Measurement of muscle microvascular oxygen pressures: compartmentalization of phosphorescent probe. Microcirculation 11:317–326
Iida H, Kurano M, Takano H, Kubota N, Morita T, Meguro K, Sato Y, Abe T, Yamazaki Y, Uno K, Takenaka K, Hirose K, Nakajima T (2007) Hemodynamic and neurohumoral responses to the restriction of femoral blood flow by KAATSU in healthy subjects. Eur J Appl Physiol 100:275–285
Burnett PE, Barrow RK, Cohen NA, Snyder SH, Sabatini DM (1998) RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci USA 95:1432–1437
Navé BT, Ouwens M, Withers DJ, Alessi DR, Shepherd PR (1999) Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem J 344:427–431
Loenneke JP, Fahs CA, Rossow LM, Abe T, Bemben MG (2012) The anabolic benefits of venous blood flow restriction training may be induced by muscle cell swelling. Med Hypotheses 78:151–154
Richardson RS, Duteil S, Wary C, Wray DW, Hoff J, Carlier PG (2006) Human skeletal muscle intracellular oxygenation: the impact of ambient oxygen availability. J Physiol 571:415–424
Richardson RS, Newcomer SC, Noyszewski EA (2001) Skeletal muscle intracellular PO2 assessed by myoglobin desaturation: response to graded exercise. J Appl Physiol 91:2679–2685
Matsumoto A, Matsumoto S, Sowers AL, Koscielniak JW, Trigg NJ, Kuppusamy P, Mitchell JB, Subramanian S, Krishna MC, Matsumoto K (2005) Absolute oxygen tension (PO2) in murine fatty and muscle tissue as determined by EPR. Magn Reson Med 54:1530–1535
Wouters BG, van den Beucken T, Magagnin MG, Koritzinsky M, Fels D, Koumenis C (2005) Control of the hypoxic response through regulation of mRNA translation. Semin Cell Dev Biol 16:487–501
Liu L, Cash TP, Jones RG, Keith B, Thompson CB, Simon MC (2006) Hypoxia-induced energy stress regulates mRNA translation and cell growth. Mol Cell 21:521–531
Arsham AM, Howell JJ, Simon MC (2003) A novel hypoxia-inducible factor-independent hypoxic response regulating mammalian target of rapamycin and its targets. J Biol Chem 278:29655–29660
Brugarolas J, Lei K, Hurley RL, Manning BD, Reiling JH, Hafen E, Witters LA, Ellisen LW, Kaelin WG (2004) Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev 18:2893–2904
Sofer A, Lei K, Johannessen CM, Ellisen LW (2005) Regulation of mTOR and cell growth in response to energy stress by REDD1. Mol Cell Biol 25:5834–5845
Wang GL, Jiang BH, Rue EA, Semenza GL (1995) Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 92:5510–5514
Breen E, Tang K, Olfert M, Knapp A, Wagner P (2008) Skeletal muscle capillarity during hypoxia: VEGF and its activation. High Alt Med Biol 9:158–166
Hardie DG (2007) AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol 8:774–785
Bolster DR, Kubica N, Crozier SJ, Williamson DL, Farrell PA, Kimball SR, Jefferson LS (2003) Immediate response of mammalian target of rapamycin (mTOR)-mediated signalling following acute resistance exercise in rat skeletal muscle. J Physiol 553:213–220
Baar K, Esser K (1999) Phosphorylation of p70 (S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol 276(1 Pt 1):C120–C127
Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD (2001) Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3:1014–1019
Fry CS, Glynn EL, Drummond MJ, Timmerman KL, Fujita S, Abe T, Dhanani S, Volpi E, Rasmussen BB (2010) Blood flow restriction exercise stimulates mTORC1 signaling and muscle protein synthesis in older men. J Appl Physiol 108:1199–1209
Terzis G, Georgiadis G, Stratakos G, Vogiatzis I, Kavouras S, Manta P, Mascher H, Blomstrand E (2008) Resistance exercise-induced increase in muscle mass correlates with p70S6 kinase phosphorylation in human subjects. Eur J Appl Physiol 102:145–152
Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, Volpi E, Rasmussen BB (2009) Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol 587(Pt 7):1535–1546
Wretman C, Lionikas A, Widegren U, Lännergren J, Westerblad H, Henriksson J (2001) Effects of concentric and eccentric contractions on phosphorylation of MAPK(erk1/2) and MAPK(p38) in isolated rat skeletal muscle. J Physiol 535:155–164
Widegren U, Ryder JW, Zierath JR (2001) Mitogen-activated protein kinase signal transduction in skeletal muscle: effects of exercise and muscle contraction. Acta Physiol Scand 172(3):227–238
Zhao Y, Bjorbaek C, Moller DE (1996) Regulation and interaction of pp90(RSK) isoforms with mitogen-activated protein kinase. J Biol Chem 271:29773–29779
Roux PP, Shahbazian D, Vu H, Holz MK, Cohen MS, Taunton J, Sonenberg N, Blenis J (2007) RAS/ERK signaling promotes site-specific ribosomal protein S6 phosphorylation via RSK and stimulates cap-dependent translation. J Biol Chem 282:14056–14064
Galcheva-Gargova Z, Derijard B, Wu IH, Davis RJ (1994) An osmosensing signal transduction pathway in mammalian cells. Science 265:806–808
Kyriakis JM, Banerjee P, Nikolakaki E, Dai T, Rubie EA, Ahmad MF, Avruch J, Woodgett JW (1994) The stress-activated protein kinase subfamily of c-Jun kinases. Nature 369:156–160
Pattwell D, McArdle A, Griffiths RD, Jackson MJ (2001) Measurement of free radical production by in vivo microdialysis during ischemia/reperfusion injury to skeletal muscle. Free Radic Biol Med 30:979–985
McArdle A, Pattwell D, Vasilaki A, Griffiths RD, Jackson MJ (2001) Contractile activity-induced oxidative stress: cellular origin and adaptive responses. Am J Physiol Cell Physiol 280:C621–C627
Korthuis RJ, Granger DN, Townsley MI, Taylor AE (1985) The role of oxygen derived free radicals in ischaemia-induced increases in skeletal muscle vascular permeability. Circ Res 57:599–609
Oredsson S, Plate G, Qvarfordt P (1991) Allopurinol—a free radical scavenger–reduces reperfusion injury in skeletal muscle. Eur J Vasc Surg 5:47–52
Qin S, Chock PB (2003) Implication of phosphatidylinositol 3-kinase membrane recruitment in hydrogen peroxide-induced activation of PI3K and Akt. Biochemistry 42:2995–3003
Glass DJ (2005) Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol 37:1974–1984
Reid MB (2005) Response of the ubiquitin-proteasome pathway to changes in muscle activity. Am J Physiol Regul Integr Comp Physiol 288:R1423–R1431
Thomas M, Langley B, Berry C, Sharma M, Kirk S, Bass J, Kambadur R (2000) Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J Biol Chem 275:40235–40243
Wehling M, Cai B, Tidball JG (2000) Modulation of myostatin expression during modified muscle use. FASEB J 14:103–110
Drummond MJ, Fujita S, Abe T, Dreyer HC, Volpi E, Rasmussen BB (2008) Human muscle gene expression following resistance exercise and blood flow restriction. Med Sci Sports Exerc 40:691–698
Takarada Y, Tsuruta T, Ishii N (2004) Cooperative effects of exercise and occlusive stimuli on muscular function in low-intensity resistance exercise with moderate vascular occlusion. Jpn J Physiol 54:585–592
Mourad JJ, Danchin N, Puel J, Gallois H, Msihid J, Safar ME, Tanaka H (2008) Cardiovascular impact of exercise and drug therapy in older hypertensives with coronary heart disease: PREHACOR study. Heart Vessels 23:20–25
Sung J, Cho SJ, Choe YH, Yoo S, Woo KG, Choi YH, Hong KP (2015) Relationship between aerobic fitness and progression of coronary atherosclerosis. Heart Vessels. doi:10.1007/s00380-015-0745-2
Acknowledgments
Dr. Yoshiaki Sato is an inventor of low-load resistance training with blood flow restriction, so-called Kaatsu training, and the owner of KAATSU Japan Co. Ltd. This work was supported by JSPS KAKENHI Grant Number 24300189 (to T.N.) and 25750288 (to T.Y.). This study was previously presented, in part, in abstract form at the Scientific Sessions, American Heart Association 2013 (Dallas, Texas, USA, 2013/11/16-11/20).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interests to disclose.
Rights and permissions
About this article
Cite this article
Nakajima, T., Yasuda, T., Koide, S. et al. Repetitive restriction of muscle blood flow enhances mTOR signaling pathways in a rat model. Heart Vessels 31, 1685–1695 (2016). https://doi.org/10.1007/s00380-016-0801-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-016-0801-6