Abstract
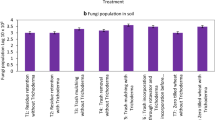
A field experiment was conducted during 2003–2005 and 2004–2006 at the Indian Institute of Sugarcane Research, Lucknow, India to study the effect of Trichoderma viride inoculation in ratoon sugarcane with three trash management practices, i.e. trash mulching, trash burning and trash removal. Trichoderma inoculation with trash mulch increased soil organic carbon and phosphorus (P) content by 5.08 Mg ha−1 and 11.7 kg ha−1 over their initial contents of 15.75 Mg ha−1 and 12.5 kg ha−1, respectively. Soil compaction evaluated as bulk density in 0- to 15-cm soil layer, increased from 1.48 Mg m−3 at ratoon initiation (in April) to 1.53 Mg m−3 at harvest (in December) due to trash burning and from 1.42 Mg m−3 at ratoon initiation (in April) to 1.48 Mg m−3 at harvest (in December) due to trash mulching. The soil basal respiration was the highest during tillering phase and then decreased gradually, thereafter with the advancement of crop growth. On an average, at all the stages of crop growth, Trichoderma inoculation increased the soil basal respiration over no inoculation. Soil microbial biomass increased in all plots except in the plots of trash burning/removal without Trichoderma inoculation. The maximum increase (40 mg C kg−1 soil) in soil microbial biomass C, however, was observed in the plots of trash mulch with Trichoderma inoculation treatment which also recorded the highest uptake of nutrient and cane yield. On an average, Trichoderma inoculation with trash mulch increased N, P and K uptake by 15.9, 4.68 and 23.6 kg ha−1, respectively, over uninoculated condition. The cane yield was increased by 12.8 Mg ha−1 with trash mulch + Trichoderma over trash removal without Trichoderma. Upon degradation, trash mulch served as a source of energy for enhanced multiplication of soil bacteria and fungi and provided suitable niche for plant–microbe interaction.

Similar content being viewed by others
References
Agarwal MP, Singh M (1986) Effect of mulches on soil temperature and sprouting of sugarcane ratoons. Int J Trop Agric 4:23–29
Black CA (1965) Methods of soil analysis, part II. American Society of Agronomy, Wisconsin, USA, p 1572
Blakemore K, Searle PL, Daly BK (1972) Methods for the chemical analysis soils. NZ Soil Bureau Report 10 A. Government Printer, Wellington
Boopathy R, Beary T, Templet PJ (2001) Microbial decomposition of post harvest sugarcane residue. Bioresour Technol 79:29–33. doi:10.1016/S0960-8524(01)00034-7
Calbrix R, Barray S, Chabrerie O, Fourrie L, Laval K (2007) Impact of organic amendments on the dynamics of soil microbial biomass and bacterial communities in cultivated land. Appl Soil Ecol 35:511–522. doi:10.1016/j.apsoil.2006.10.007
Cerri CC, Bernoux M, Cerri CEP, Feller C (2004) Carbon cycling and sequestration opportunities in South America: the case of Brazil. Soil Use Manage 20:248–254. doi:10.1079/SUM2004237
Chet I (1987) Trichoderma, application, mode of action and potential as biological control agent of soil borne plant pathogenic fungi. In: Chet I (ed) Innovative approach to plant disease control. Wiley, New York, pp 137–160
Chet I, Inbar J, Hodar Y (1997) Fungal antagonists and mycoparasites. In: Wicklow S (ed) Mycota environmental and microbial relationships, vol. IV. Springer, Berlin, pp 165–184
Cocharan WG, Cox GM (1957) Experimental designs, 2nd edn. Wiley, New York, USA, pp 463–66
Cong PT, Merckx R (2005) Improving phosphorous availability in two upland soils of Vietnam using Tithonia diversifolia. Plant Soil 269:11–23. doi:10.1007/s11104-004-1791-1
Doran JW, Parkin TB (1994) Defining and assessing soil quality. In: Doran JW et al. (ed). Defining soil quality for a sustainable environment. SSSA Spec. Publ. No: 35. Madison, WI. p. 3–21.
Garside AL (1997) Yield decline research in the Australian sugar industry. Proc S Afr Sugar Technol Assoc 71:3–18
Harman GE (2000) Myth and dogmas of biocontrol changes in perceptions derived from research on Trichoderma harzianum T-22. Plant Dis 84:377–393. doi:10.1094/PDIS.2000.84.4.377
Harman GE, Bjorkman T (1998) Potential and existing uses of Trichoderma and Gliocladium for plant disease control and plant growth enhancement. In: Harman GE, Kubicek CP (eds) Trichoderma and Gliocladium, vol. II. Taylor and Francis, London, pp 229–265
Harman GE, Lumsden RD (1990) Biological disease control. In: Lynch JM (ed) The rhizoshpere. Wiley Interscience, New York, pp 259–280
Harman GE, Howell CR, Viderbo A, Chet I, Loreto M (2001) Trichoderma spp. Opportunistic avirulent plant symbiont. Nat Rev Microbiol 2:43–56. doi:10.1038/nrmicro797
Howell CR, Hanoon LE, Stipanovic RD, Puckhaber SI (2000) Induction of terpenoid synthesis in cotton roots and control of Rhizoctonia solani by seed treatment with Trichoderma viride. Phytopathology 90:248–252. doi:10.1094/PHYTO.2000.90.3.248
Hunsigi G (2001) Ratoon management. In: Sugarcane in agriculture and industry. Prism Books, Bangalore, p 217
Jackson ML (1973) Soil chemical analysis. Prentice-Hall of India Pvt. Ltd., New Delhi, p 183
Jenkinson DS, Ladd JN (1981) Microbial biomass in soil: measurement and turnover. In: Paul EA, Ladd JN (eds) Soil biochemistry, Vol V. Marcel Dekker, New York, pp 415–471
Jenkinson DS, Powlson DS (1976) The effects of biocidal treatment on metabolism in soil: I. Fumigation with chloroform. Soil Biol Biochem 8:167–177. doi:10.1016/0038-0717(76)90001-8
Ousley MA, Lynch JM, Whipps JM (1994) Potential of Trichoderma as consistent plant growth stimulators. Biol Fertil Soils 17:85–90. doi:10.1007/BF00337738
Patra DD, Ram M, Singh DV (1993) Influence of straw mulching on fertilizer nitrogen use efficiency, moisture conservation and herb and essential oil yield of Japanese mint. Fertil Res 34:135–139. doi:10.1007/BF00750108
Piper CS (1966) Soil and plant analysis. University of Adelaide, Australia
Razafimbelo T, Bernard Barthès B, Larré-Larrouy MC, De Luca EF, Laurent JY, Cerri CC, Feller C (2006) Effect of sugarcane residue management (mulching versus burning) on organic matter in a clayey oxisol from southern Brazil. Agric Ecosyst Environ 115:285–289. doi:10.1016/j.agee.2005.12.014
Saravanan A, Mahendran PP (2003) Enriches organic wastes—the challenging perspective in agriculture. In: Subramaniam S, Saravanan SA, Santhy P, Sheeba S, Jayalkhmi M (eds) Lecture on short course on Eco-friendly Recycling of organic and Industrial Wastes for sustainable soil health, November 5–14, 2003. T.N. Agricultural University, Madurai, India, pp 35–50
Shukla SK, Yadav RL, Suman A, Singh PN (2008) Improving rhizospheric environment and sugarcane ratoon yield through bioagents amended farm yard manures in udic Ustochrept soil. Soil Tillage Res 99:158–168. doi:10.1016/j.still.2008.02.007
Singh M, Sharma RM (1991) Microbial population and decomposition of sugarcane trash at different relative humidities. J Indian Soc Soil Sci 39:189–190
Singh KP, Suman A, Singh PN (2007) Improving quality of sugarcane growing soils by organic amendments under sub-tropical climatic conditions of India. Biol Fertil Soils 44:367–376. doi:10.1007/s00374-007-0216-8
Sinha MK, Sinha DP, Sinha H (1977) Organic matter transformations in soil. V. Kinetics of carbon. and nitrogen mineralization in soils amended with different organic materials. Plant Soil 46:579–590. doi:10.1007/BF00015917
Speir TW, Horswel J, Mclaren RG, Fietje G, Vanschalk PP (2004) Composted biosolids enhance fertility of a sandy loam soil under dairy pasture. Biol Fertil Soils 40:349–358. doi:10.1007/s00374-004-0787-6
Sparling GP, Schipper LA, McLeod M, Basher L, Rijkse W (1998) Trialing soil quality indicators for state of the environment monitoring. Research report 1997/1998. Unpublished Landcare Research Contract Report LC9798/141 for the Sustainable Management Fund, Ministry for the Environment Project 5001. Landcare Research, Lincoln, New Zealand
Sundara B, Tripathi BK (1989) Available N changes and N balance under multi ratooning of sugarcane varieties in tropical vertisol. Proceedings 23rd International Society of Sugarcane Technologists, pp 80–88
Verma RS (2002) Sugarcane ratoon management. International Book Distributing Co. Pvt. Ltd., Lucknow, p 202
Ward Lee DA (1992) A comparative assessment of factors which influence microbial biomass carbon and nitrogen levels in soil. Biol Rev Camb Philos Soc 67:321–358. doi:10.1111/j.1469-185X.1992.tb00728.x
Yadav RL, Prasad SR (1992) Conserving the organic matter content of the soil to sustain sugarcane yield. Exp Agric 28:57–62. doi:10.1017/S0014479700023012
Yadav RL, Prasad SR, Singh R, Srivastava VK (1994) Recycling sugarcane trash to conserve soil organic for sustaining yields of successive ratoon crops in sugarcane. Bioresour Technol 49:231–235. doi:10.1016/0960-8524(94)90045-0
Yedidia I, Srivatka A, Kapulnik Y, Chet I (2001) Effect of Trichoderma harzianum on uptake of microelements and increased growth response of cucumber plants. Plant Soil 235:235–242. doi:10.1023/A:1011990013955
Zaller JG, Kopke U (2004) Effects of traditional and biodynamic farmyard manure amendments on yields, soil chemical and biological properties in a long term experiment. Biol Fertil Soils 40:222–229. doi:10.1007/s00374-004-0772-0
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yadav, R.L., Shukla, S.K., Suman, A. et al. Trichoderma inoculation and trash management effects on soil microbial biomass, soil respiration, nutrient uptake and yield of ratoon sugarcane under subtropical conditions. Biol Fertil Soils 45, 461–468 (2009). https://doi.org/10.1007/s00374-009-0352-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-009-0352-4