Abstract
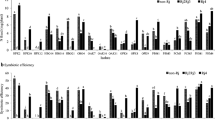
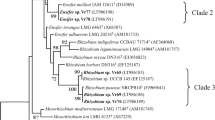
A study on the diversity, phylogeny, and host specificity of soybean (Glycine max L.) and peanut (Arachis hypogaea L.) bradyrhizobia was conducted based on the 16S ribosomal RNA (rRNA) restriction fragment length polymorphisms (RFLPs), 16S rRNA sequencing, and 16S–23S rRNA intergenetic spacer (IGS) RFLP assays. Based on 16S rRNA RFLP assay, tested bradyrhizobia were divided into five genotypes, which could be further clustered into five groups by IGS RFLP assays. According to the 16S rRNA sequencing, strains of IGS-II, IV, and V were phylogenetically related to Bradyrhizobium liaoningense, Bradyrhizobium japonicum, and Bradyrhizobium elkanii, while strains of IGS-Ic and IGS-III related to Bradyrhizobium yuanmingense and Bradyrhizobium canariense, respectively. All isolates could crossly nodulate Phaseolus vulgaris, forming small white nodules. Strains of IGS-II originally isolated from peanut could efficiently nodulate Glycine soja, and two strains isolated from soybean could also nodulate peanut.



Similar content being viewed by others
References
Abaidoo RC, Keyser HH, Singleton PW, Borthakur D (2000) Bradyrhizobium spp. (TGx) isolates nodulating the new soybean cultivars in Africa are diverse and distinct from bradyrhizobia that nodulate North American soybeans. Int J Syst Evol Microbiol 50:225–234
Anandham R, Sridar R, Nalayini P, Poonguzhali S, Madhaiyan M, Sa T (2007) Potential for plant growth promotion in groundnut (Arachis hypogaea L.) cv. ALR-2 by co-inoculation of sulfur-oxidizing bacteria and Rhizobium. Microbiol Res 162:139–153
Barrera LL, Trujillo ME, Goodfellow M, Garcia FJ, Hernamdez-Lucas L, Davila G, van Berkum P, Martinez-Romero E (1997) Biodiversity of bradyrhizobia nodulating Lupinus spp. Int J Syst Bacteriol 47:1086–1091
Chaintreuil C, Giraud E, Prin Y, Lorquin J, Ba A, Gillis M, de Lajudie P, Dreyfus B (2000) Photosynthetic bradyrhizobia are natural endophytes of the African wild rice Oryza breviligulata. Appl Environ Microbiol 66:5437–5447
Cheng GJ, Li YG, Zhou JC (2006) Cloning and identification of opa22, a new gene involved in nodule formation by Mesorhizobium huakuii. FEMS Microbiol Lett 257:152–157
de Fátima Loureiro M, Kaschuk G, Alberton O, hungria M (2007) Soybean (Glycine max (L.) Merrill) rhizobial diversity in Brazilian oxisols under various soil, cropping, and inoculation managements. Biol Fertil Soils 43:665–674
Deya AAM, Odelson DA, Hickey RF, Tidje JM (1995) Bacterial community fingerprinting of amplified 16S and 16S–23S ribosomal DNA gene sequences and restriction endonuclease analysis (ARDRA). In: Akkermans DL, van Elsas JD, de Bruijn FJ (eds) Molecular microbial ecology manual. vol. 3.3.2. Kluwer Academic, Dordrecht, pp 1–8
Freiberg C, Fellay R, Bairoch A, Broughton WJ, Rosenthal A, Perret X (1997) Molecular basis of symbiosis between Rhizobium and legumes. Nature 387:394–401
Han SZ, Wang ET, Chen WX (2005) Diverse bacteria isolated from root nodules of Phaseolus vulgaris and species within the genera Campylotropis and Cassia grown in China. Syst Appl Microbiol 28:265–276
Jordan DC (1982) Transfer of Rhizobium japonicum Buchanan 1980 to Bradyrhizobium gen. nov., a genus of slow-growing root nodule bacteria from leguminous plants. Int J Syst Bacteriol 32:136–139
Kimura M (1980) A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Kuykendall LD, Saxena B, Devine EE, Udell SE (1992) Genetic diversity in Bradyrhizobium japonicum Jordan 1982, and a proposal for Bradyrhizobium elkanii sp. nov. Can J Microbiol 38:501–505
Mantel N (1967) The detection of disease clustering and a generalized regression approach. Cancer Res 27:209–220
Ormeño-Orrillo E, Vinuesa P, Zúñiga-Dávila D, Martínez-Romero E (2006) Molecular diversity of native bradyrhizobia isolated from lima bean (Phaseolus lunatus L.) in Peru. Syst Appl Microbiol 29:253–262
Perry LG, Alford ER, Horiuchi J, Paschke MW, Vivanco JM (2007) Chemical signals in the rhizosphere root–root and root–microbe communication. In: Pinton R, Varanini Z, Nannipieri P (eds) The rhizosphere biochemistry and organic substances at the soil–plant interface. 2nd edn. CRC, Boca Raton, pp 297–330
Pueppke SG, Broughton WJ (1999) Rhizobium sp. strain NGR234 and R. fredii USDA257 share exceptionally broad, nested host ranges. Mol Plant Microbe Interact 12:293–318
Rivas R, Willems A, Palomo JL, Garcia-Benavides P, Mateos PF, Martinez-Molina E, Gillis M, Velazquez E (2004) Bradyrhizobium betae sp. nov. isolated from roots of Beta vulgaris affected by tumour-like deformations. Int J Syst Evol Microbiol 54:1271–1275
Rodríguez-Navarro DN, Camacho M, Leidi EO, Rivas R, Velázquez E (2004) Phenotypic and genotypic characterization of rhizobia from diverse geographical origin that nodulate Pachyrhizus species. Syst Appl Microbiol 27:737–745
Safronova V, Chizhevskaya E, Bullitta S, Andronov E, Belimov A, Charles TC, Lindström K (2007) Presence of a novel 16S-23S rRNA gene intergenic spacer insert in Bradyrhizobium canariense strains. FEMS Microbiol Lett 269:207–212
Sameshima R, Isawa T, Sadowsky MJ, Hamada T, Kasai H, Shutsrirung A, Mitsui H, Minamisawa K (2003) Phylogeny and distribution of extra-slow-growing Bradyrhizobium japonicum harboring high copy numbers of RSa, RSb and IS1631. FEMS Microbiol Ecol 44:191–202
So RB, Ladha JK, Young JP (1994) Photosynthetic symbionts of Aeschynomene spp. Form a cluster with bradyrhizobia on the basis of fatty acid and rRNA analyses. Int J Syst Bacteriol 44:392–403
Stepkowski T, Moulin L, Krzyzańska A, McInnes A, Law IJ, Howieson J (2005) European origin of Bradyrhizobium populations infecting lupins and serradella in soils of Western Australia and South Africa. Appl Environ Microbiol 71:7041–752
Urtz BE, Elkan GH (1996) Genetic diversity among Bradyrhizobium isolates that effectively nodulate peanut (Arachis hypogaea). Can J Microbiol 42:1121–1130
van Berkum P, Fuhrmann JJ (2000) Evolutionary relationships among the soybean bradyrhizobia reconstructed from 16S rRNA gene and internally transcribed spacer region sequence divergence. Int J Syst Evol Microbiol 50:2165–2172
van Rossum D, Schuurmans FP, Gillis M, Tcha AM, van Verseveld HW, Stouthamer AH, Boogerd FC (1995) Genetic and phenetic analyses of Bradyrhizobium strains nodulating peanut (Arachis hypogaea L.) roots. Appl Environ Microbiol 61:1599–1609
Vincent JM (1970) A manual for the practical study of root-nodule bacteria. International biological programme handbook. Blackwell Scientific, Oxford, pp 73–97
Vinuesa P, Rademaker JLW, de Bruijn FJ, Werner D (1998) Genotypic characterization of Bradyrhizobium strains nodulating endemic woody legumes of the Canary islands by PCR-restriction fragment length polymorphism analysis of genes encoding 16S rRNA (16S rDNA) and 16S–23S rDNA intergenic spacers, repetitive extragenic palindromic PCR genomic fingerprinting, and partial 16S rDNA sequencing. Appl Environ Microbiol 64:2096–2104
Vinuesa P, Leon-Barrios M, Silva C, Willems A, Jarabo-Lorenzo A, Perez-Galdona R, Werner D, Martinez-Romero E (2005) Bradyrhizobium canariense sp. nov., an acid-tolerant endosymbiont that nodulates endemic genistoid legumes (Papilionoideae: Genisteae) from the Canary Islands, along with Bradyrhizobium japonicum bv. genistearum, Bradyrhizobium genospecies alpha and Bradyrhizobium genospecies beta. Int J Syst Evol Microbiol 55:569–575
Willems A, Doignon-Bourcier F, Coopman R, Hoste B, de Lajudie P, Gillis M (2000) AFLP fingerprint analysis of Bradyrhizobium strains isolated from Faidherbia albida and Aeschynomene species. Syst Appl Microbiol 23:137–147
Wilson K (1989) Preparation of genomic DNA from bacteria. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Srtruhl K (eds) Current protocols in molecular Biology. Greene Publishing Associates/Wiley-Interscience, New York, pp 2.4.1–2.4.5
Xu LM, Ge C, Cui Z, Li J, Fan H (1995) Bradyrhizobium liaoningense sp. nov., isolated from the root nodules of soybeans. Int J Syst Bacteriol 45:706–711
Yang JK, Xie FL, Zou J, Zhou Q, Zhou JC (2005) Polyphasic characteristics of bradyrhizobia isolated from nodules of peanut (Arachis hypogaea) in China. Soil Biol Biochem 37:141–153
Yang JK, Zhang WT, Yuan TY, Zhou JC (2006) Genotypic characteristics of the rrn operon and genome of indigenous soybean bradyrhizobia in cropping zones of China. Can J Microbiol 9:968–976
Yao ZY, Kan FL, Wang ET, Wei GH, Chen WX (2002) Characterization of rhizobia that nodulate legume species of the genus Lespedeza and description of Bradyrhizobium yuanmingense sp. nov. Int J Syst Evol Microbiol 52:2219–2230
Zhang X, Nick G, Kaijalainen S, Terefewirjm Z, Paulin L, Tighe SW, Graham PH, Lindstrom K (1999) Phylogeny an diversity of Bradyrhizobium strains isolated from the root nodules of peanut (Arachis hypogaea) in Sichuan, China. Syst Appl Microbiol 22:378–386
Acknowledgements
This work was granted by Chinese High-tech Developing Program 2007AA05Z417, Chinese Microbe Resource Project 2005DKA21208-6 and Grant of State Key Laboratory of Agricultural Microbiology, HAU, China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, J.K., Zhou, J.C. Diversity, phylogeny and host specificity of soybean and peanut bradyrhizobia. Biol Fertil Soils 44, 843–851 (2008). https://doi.org/10.1007/s00374-008-0269-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-008-0269-3