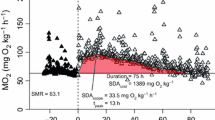
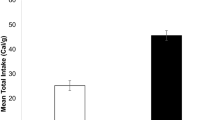
Abstract
Shallow coastal and estuarine habitats function as nurseries for many juvenile fish. In this comparative study, metabolic profiles of two New Zealand finfish, snapper (Chrysophrys auratus) and yellow-eyed mullet—YEM (Aldrichetta forsteri) that as juveniles share the same temperate coastal environments, were examined. Metabolic parameters (routine and maximum metabolic rates, and specific dynamic action—SDA) were investigated at a set of temperatures (13, 17, 21 °C) within the range juveniles both species experience annually. SDA was also determined for a range of different feed rations to investigate the effects of meal size on postprandial metabolic response. Temperature was a strong modulator of snapper and YEM metabolic profile (routine and maximum metabolic rates, and absolute and factorial aerobic scope). Metabolic rates increased with temperature in both species as did absolute scope in YEM, though for snapper, it was only greater at the highest temperature. Factorial scope behaved in the same fashion for the two species, being greatest at 13 °C. Both absolute and factorial scope were ~ twofold greater in YEM than in snapper across the entire temperature range. Temperature also affected SDA response in snapper, while in YEM, SDA parameters were largely unaffected when temperature increased from 17 to 21 °C. Snapper were able to consume a large range of meal sizes (0.5–3.0% body mass—BM) with meal sizes > 1% BM having a pronounced effect on numerous SDA parameters, whereas mullet appeared to consume more limited ration sizes (≤ 1.0% BM). In both species, rations ≤ 1% BM produced similar changes in SDA parameters identifying comparable digestive bio-energetics. Overall, our metabolic characterisations demonstrate that both species can adjust to the variable temperate environmental temperatures and manage the energetic costs of digestion and feed assimilation. Yet, despite these general similarities, YEM’s greater aerobic scope may point to better physiological adaptation to the highly variable temperate coastal environment than were observed in snapper.




Similar content being viewed by others
References
Armstrong JD, Priede IG, Lucas MC (1992) The link between respiratory capacity and changing metabolic demands during growth of northern pike, Esox lucius L. J Fish Biol 41(Suppl. B):65–75
Bin K, Xian WW (2005) Feeding level-scaled retention efficiency, growth and energy partitioning of a marine detritivorous fish, redlip mullet (Liza haematocheila T. & S.). Aquac Res 36:906–911
Blaber SJM, Blaber TG (1980) Factors affecting the distribution of juvenile estuarine and inshore fish. J Fish Biol 17:143–162
Blazka P, Volf M, Cepela M (1960) A new type of respirometer for the determination of the metabolism of fish in an active state. Physiol Bohemoslov 9:553–558
Boyce SJ, Clarke A (1997) Effect of body size and ration on specific dynamic action in the Antarctic plunderfish, Harpagifer antarcticus Nybelin 1947. Physiol Zool 70:679–690
Brown EJ, Bruce M, Pether S, Herbert NA (2011) Do swimming fish always grow fast? Investigating the magnitude and physiological basis of exercise-induced growth in juvenile New Zealand yellowtail kingfish Seriola lalandi. Fish Physiol Biochem 37:327
Chabot D, McKenzie D, Craig J (2016a) Metabolic rate in fishes: definitions, methods and significance for conservation physiology. J Fish Biol 88:1–9
Chabot D, Steffensen JF, Farrell A (2016b) The determination of standard metabolic rate in fishes. J Fish Biol 88:81–121
Chabot D, Koenker R, Farrell A (2016c) The measurement of specific dynamic action in fishes. J Fish Biol 88:152–172
Chakraborty S, Ross L, Ross B (1992) Specific dynamic action and feeding metabolism in common carp, Cyprinus carpio L. Comp Biochem Physiol A Physiol 103:809–815
Chubb C, Potter IC, Grant C, Lenanton R, Wallace J (1981) Age, stucture, growth rates and movements of sea mullet, Mugil cephalus L., and Yellow-eye Mullet, Aldrichetta forsteri (Valenciennes), in the Swan-Avon river system, Western Australia. Mar Freshwater Res 32:605–628
Claireaux G, Webber D, Lagardère JP, Kerr S (2000) Influence of water temperature and oxygenation on the aerobic metabolic scope of Atlantic cod (Gadus morhua). J Sea Res 44:257–265
Clark TD, Seymour RS (2006) Cardiorespiratory physiology and swimming energetics of a high-energy-demand teleost, the yellowtail kingfish (Seriola lalandi). J Exp Biol 209:3940–3951. https://doi.org/10.1242/jeb.02440
Clark TD, Jeffries KM, Hinch SG, Farrell AP (2011) Exceptional aerobic scope and cardiovascular performance of pink salmon (Oncorhynchus gorbuscha) may underlie resilience in a warming climate. J Exp Biol 214:3074–3081
Clark TD, Donaldson MR, Pieperhoff S, Drenner SM, Lotto A, Cooke SJ et al (2012) Physiological benefits of being small in a changing world: responses of coho salmon (Oncorhynchus kisutch) to an acute thermal challenge and a simulated capture event. PLoS ONE 7:e39079
Clark TD, Sandblom E, Jutfelt F (2013) Aerobic scope measurements of fishes in an era of climate change: respirometry, relevance and recommendations. J Exp Biol 216:2771–2782
Cossins A, Bowler K (1987) Rate compensations and capacity adaptations. In: Cossins AR (ed) Temperature biology of animals. Springer, Dordrecht, pp 155–203
Coubrough S, Jerrett A, Goodwin E (2004) Feeding behaviour of yellow-eyed mullet (Aldrichetta forsteri) and snapper (Pagrus auratus) in relation to ambient changes in water temperature and light. Crop Food Res Confid Rep 1186:1–24
Coxon SE (2014) The exercise physiology of snapper (Pagrus auratus): implications for the better commercial harvesting of an iconic New Zealand finfish. Dissertation, University of Canterbury
Curtis TD, Shima JS (2005) Geographic and sex-specific variation in growth of yellow-eyed mullet, Aldrichetta forsteri, from estuaries around New Zealand. N Z J Mar Fresh 39:1277–1285
Davison W, Franklin CE, Mckenzie JC, Dougan MC (1992) The effects of acute exposure to the water soluble fraction of diesel fuel oil on survival and metabolic rate of an Antarctic fish (Pagothenia borchgrevinki). Comp Biochem Physiol C Comp Pharmacol Toxicol 102:185–188
Du Preez HH, Strydom W, Winter P (1986) Oxygen consumption of two marine teleosts, Lithognathus mormyrus (linnaeus, 1758) and Lihognathus lithognathus (cuvier, 1830) (Teleosti: Sparidae). Comp Biochem Physiol A 85:313–331
Elliott M, Hemingway KL (2008) Fishes in estuaries. Blackwell, London
Elliott M, Quintino V (2007) The estuarine quality paradox, environmental homeostasis and the difficulty of detecting anthropogenic stress in naturally stressed areas. Mar Pollut Bull 54:640–645
Farrell AP (2016) Pragmatic perspective on aerobic scope: peaking, plummeting, pejus and apportioning. J Fish Biol 88:322–343
Farrell AP, Hinch SG, Cooke SJ, Patterson DA, Crossin GT, Lapointe M, Mathes MT (2008) Pacific salmon in hot water: applying aerobic scope models and biotelemetry to predict the success of spawning migrations. Physiol Biochem Zool 81:697–708
Flikac T, Cook DG, Davison W, Jerrett A (in review) Seasonal growth dynamics and maximum potential growth rates of Australasian snapper (Chrysophrys auratus) and yellow-eyed mullet (Aldrichetta forsteri). Aquacult Rep
Flowerdew M, Grove D (1980) An energy budget for juvenile thick-lipped mullet, Crenimugil labrosus (Risso). J Fish Biol 17:395–410
Forster ME (1990) Confirmation of the low metabolic rate of hagfish. Comp Biochem Physiol A Comp Physiol 96:113–116
Frederich M, Pörtner HO (2000) Oxygen limitation of thermal tolerance defined by cardiac and ventilatory performance in spider crab, Maja squinado. Am J Physiol Regul Integr Comp Physiol 279:R1531–R1538
Frisk M, Steffensen JF, Skov PV (2013) The effects of temperature on specific dynamic action and ammonia excretion in pikeperch (Sander lucioperca). Aquaculture 404:65–70
Fry FEJ (1971) The effect of environmental factors on the physiology of fish. In: Hoar WS, Randall DJ (eds) Fish physiology. Academic Press Inc, New York, pp 1–87
Fry FEJ, Hochachka PW (1970) Fish. In: Whitton GC (ed) Comparative physiology of thermoregulation, 1st edn. Academic Press, New York, pp 79–130
Fu S, Xie X, Cao Z (2005) Effect of feeding level and feeding frequency on specific dynamic action in Silurus meridionalis. J Fish Biol 67:171–181
Fu S, Cao Z, Peng J (2006) Effect of meal size on postprandial metabolic response in Chinese catfish (Silurus asotus Linnaeus). J Comp Physiol B 176:489–495
Fulton CJ, Johansen JL, Steffensen JF (2013) Energetic extremes in aquatic locomotion by coral reef fishes. PLoS ONE 8:e54033. https://doi.org/10.1371/journal.pone.0054033
Glazier DS (2005) Beyond the ‘3/4-power law’: variation in the intra-and interspecific scaling of metabolic rate in animals. Biol Rev 80:611–662
Glazier DS (2009) Activity affects intraspecific body-size scaling of metabolic rate in ectothermic animals. J Comp Physiol B 179:821–828
Gnaiger E (1983) Calculation of energetic and biochemical equivalents of respiratory oxygen consumption. In: Forstner H, Gnaiger E (eds) Polarographic oxygen sensors. Springer, New York, pp 337–345
Guinea J, Fernandez F (1991) The effect of SDA, temperature and daily rhythm on the energy metabolism of the mullet Mugil saliens. Aquaculture 97:353–364
Guinea J, Fernandez F (1997) Effect of feeding frequency, feeding level and temperature on energy metabolism in Sparus aurata. Aquaculture 2:125–142
Hammer C (1995) Fatigue and exercise tests with fish. Comp Biochem Physiol A Physiol 112:1–20
Hartill B, Morrison M, Smith M, Boubée J, Parsons D (2003) Diurnal and tidal movements of snapper (Pagrus auratus, Sparidae) in an estuarine environment. Mar Fresh Res 548:931–940
Huang Q, Zhang Y, Liu S, Wang W, Luo Y (2013) Intraspecific scaling of the resting and maximum metabolic rates of the crucian carp (Carassius auratus). PLoS ONE 8:e82837
Ibarz A, Fernández-Borràs J, Blasco J, Gallardo MÁ, Sánchez J (2003) Oxygen consumption and feeding rates of gilthead sea bream (Sparus aurata) reveal lack of acclimation to cold. Fish Physiol Biochem 29:313–321
Ibarz A, Blasco J, Sala-Rabanal M, Gallardo MÁ, Redondo A, Fernández-Borràs J (2007) Metabolic rate and tissue reserves in gilthead sea bream (Sparus aurata) under thermal fluctuations and fasting and their capacity for recovery. Can J Fish Aquat Sci 64:1034–1042
Ibarz A, Padrós F, Gallardo MÁ, Fernández-Borràs J, Blasco J, Tort L (2010) Low-temperature challenges to gilthead sea bream culture: review of cold-induced alterations and ‘Winter Syndrome’. Rev Fish Biol Fish 20:539–556
Jobling M (1981) The influences of feeding on the metabolic rate of fishes: a short review. J Fish Biol 18:385–400
Jobling M (1983) Towards an explanation of specific dynamic action (SDA). J Fish Biol 23:549–555
Jobling M (1994) Fish bioenergetics. Chapman & Hall, London
Jobling M (1997) Temperature and growth: modulation of growth rate via temperature change. In: Wood CM, McDonald DG (eds) Global warming: implications for freshwater and marine fish. Cambridge University Press, Cambridge, pp 225–253
Jobling M, Davies PS (1980) Effects of feeding on metabolic rate, and the specific dynamic action in plaice, Pleuronectes platessa L. J Fish Biol 16:629–638
Jordan AD, Steffensen JF (2007) Effects of ration size and hypoxia on specific dynamic action in the cod. Physiol Biochem Zool 80:178–185
Khan J, Pether S, Bruce M, Walker S, Herbert N (2015) The effect of temperature and ration size on specific dynamic action and production performance in juvenile hapuku (Polyprion oxygeneios). Aquaculture 437:67–74
Killen SS, Costa I, Brown JA, Gamperl AK (2007) Little left in the tank: metabolic scaling in marine teleosts and its implications for aerobic scope. Proc R Soc Lond B Biol Sci 274:431–438
Kingsolver JG, Huey RB (2008) Size, temperature, and fitness: three rules. Evol Ecol Res 10:251–268
Kofuji PYM, Akimoto A, Hosokawa H, Masumoto T (2005) Seasonal changes in proteolytic enzymes of yellowtail Seriola quinqueradiata (Temminck & Schlegel; Carangidae) fed extruded diets containing different protein and energy levels. Aquac Res 36:696–703
Lefrancois C, Claireaux G (2003) Influence of ambient oxygenation and temperature on metabolic scope and scope for heart rate in the common sole Solea solea. Mar Ecol Prog Ser 259:273–284
Luo Y, Xie X (2008) Specific dynamic action in two body size groups of the southern catfish (Silurus meridionalis) fed diets differing in carbohydrate and lipid contents. Fish Physiol Biochem 34:465
McCue M (2006) Specific dynamic action: a century of investigation. Comp Biochem Physiol A Mol Integr Physiol 144:381–394
McDowall R (1978) New Zealand freshwater fishes: a guide and natural history. Heinemann, Auckland
Milinkovitch T, Lucas J, Le Floch S, Thomas-Guyon H, Lefrançois C (2012) Effect of dispersed crude oil exposure upon the aerobic metabolic scope in juvenile golden grey mullet (Liza aurata). Mar Pollut Bull 64:865–871
Nagelkerken I, Sheaves M, Baker R, Connolly RM (2015) The seascape nursery: a novel spatial approach to identify and manage nurseries for coastal marine fauna. Fish Fish 16:362–371
Norin T, Clark TD (2016) Measurement and relevance of maximum metabolic rate in fishes. J Fish Biol 88:122–151
Owen SF (2001) Meeting energy budgets by modulation of behaviour and physiology in the eel (Anguilla anguilla L.). Comp Biochem Physiol A Mol Integr Physiol 128:629–642
Parsons D, Sim-Smith C, Cryer M, Francis M, Hartill B, Jones E et al (2014) Snapper (Chrysophrys auratus): a review of life history and key vulnerabilities in New Zealand. N Z J Mar Fresh 48:256–283
Paulin CD (1990) Pagrus auratus, a new combination for the species known as “snapper” in Australasian waters (Pisces: Sparidae). N Z J Mar Fresh 24:259–265
Pérez-Casanova JC, Lall SP, Gamperl AK (2010) Effects of dietary protein and lipid level, and water temperature, on the post-feeding oxygen consumption of Atlantic cod and haddock. Aquac Res 41:198–209
Pörtner HO (2001) Climate change and temperature-dependent biogeography: oxygen limitation of thermal tolerance in animals. Naturwissenschaften 88:137–146
Pörtner HO (2010) Oxygen-and capacity-limitation of thermal tolerance: a matrix for integrating climate-related stressor effects in marine ecosystems. J Exp Biol 213:881–893
Pörtner HO, Farrell AP (2008) Physiology and climate change. Science 322:690–692
Pörtner HO, Knust R (2007) Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 315:95–97
Powell MK, Mansfield-Jones J, Gatten RE Jr (1999) Specific dynamic effect in the horned frog Ceratophrys cranwelli. Copeia 1999:710–717
Reidy S, Nelson J, Tan Y, Kerr S (1995) Post-exercise metabolic rate in Atlantic cod and its dependence upon the method of exhaustion. J Fish Biol 47:377–386
Requena A, Fernandez-Borras J, Planas J (1997) The effects of a temperature rise on oxygen consumption and energy budget in gilthead sea bream. Aquac Int 5:415–426
Reynolds WW, Casterlin ME (1979) Behavioral thermoregulation and the “final preferendum” paradigm. Am Zool 19:211–224
Robertson R, Meagor J, Taylor E (2002) Specific dynamic action in the shore crab, Carcinus maenas (L.), in relation to acclimation temperature and to the onset of the emersion response. Physiol Biochem Zool 75:350–359
Robinson E, Davison W (2008) The Antarctic notothenioid fish Pagothenia borchgrevinki is thermally flexible: acclimation changes oxygen consumption. Polar Biol 31:317–326
Ross L, McKinney R, Cardwell S, Fullarton J, Roberts S, Ross B (1992) The effects of dietary protein content, lipid content and ration level on oxygen consumption and specific dynamic action in Oreochromis niloticus L. Comp Biochem Physiol A 103:573–578
Secor SM (2009) Specific dynamic action: a review of the postprandial metabolic response. J Comp Physiol B 179:1–56
Secor SM, Wooten JA, Cox CL (2007) Effects of meal size, meal type, and body temperature on the specific dynamic action of anurans. J Comp Physiol B 177:165–182
Soofiani N, Hawkins A (1982) Energetic costs at different levels of feeding in juvenile cod, Gadus morhua L. J Fish Biol 21:577–592
Soofiani N, Priede IG (1985) Aerobic metabolic scope and swimming performance in juvenile cod, Gadus morhua L. J Fish Biol 26:127–138
Taylor PR, Paul LJ (1998) A summary of the biology, recreational and commercial landings, and stock assessment of yellow-eyed mullet, Aldrichetta forsteri (Cuvier and Valenciennes, 1836) (Mugiloidei: Mugilidae). New Zeal Fisheries Assessment Research Document 98/17 NIWA Wellington
Tirsgaard B, Svendsen JC, Steffensen JF (2015) Effects of temperature on specific dynamic action in Atlantic cod Gadus morhua. Fish Physiol Biochem 41:41–50
Usmar N (2012) Ontogenetic diet shifts in snapper (Pagrus auratus: Sparidae) within a New Zealand estuary. N Z J Mar Fresh 46:31–46
Vagner M, Zambonino-Infante JL, Mazurais D, Imbert-Auvray N, Ouillon N, Dubillot E et al (2014) Reduced n-3 highly unsaturated fatty acids dietary content expected with global change reduces the metabolic capacity of the golden grey mullet. Mar Biol 161:2547–2562
Vanella FA, Boy CC, Lattuca ME, Calvo J (2010) Temperature influence on post-prandial metabolic rate of sub-Antarctic teleost fish. Comp Biochem Physiol A Mol Integr Physiol 156:247–254
Wallace JH (1976) The food of the fish of the Blackwood River Estuary. Environmental Protection Authority. Perth, Western Australia. Environmental Protection Authority Technical Report 5. P 8
Wang T, Zaar M, Arvedsen S, Vedel-Smith C, Overgaard J (2002) Effects of temperature on the metabolic response to feeding in Python molurus. Comp Biochem Physiol A Mol Integr Physiol 133:519–527
Wang Q, Wang W, Huang Q, Zhang Y, Luo Y (2012) Effect of meal size on the specific dynamic action of the juvenile snakehead (Channa argus). Comp Biochem Physiol A Mol Integr Physiol 161:401–405
Xie S, Cui Y, Yang Y, Liu J (1997) Bioenergetics of Nile tilapia, Oreochromis niloticus: effects of food ration size on metabolic rate. Asian Fish Sci 10:155–162
Acknowledgements
This work was supported by the New Zealand Crown Research Institute for Plant and Food Research Limited (PFR) special grant “Wildfish 2030” (Contract # C11X1203). We are thankful to PFR for providing of state-of-the-art facilities including experimental animals for this work. The work was conducted also in collaboration with School of Biological Sciences, University of Canterbury, New Zealand.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
All experiments were conducted in accordance with the University of Canterbury Animal Ethics Committee (Ref: 2014/18R). All methodologies were in compliance with approved guidelines.
Additional information
Communicated by B. Pelster.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Flikac, T., Cook, D.G. & Davison, W. The effect of temperature and meal size on the aerobic scope and specific dynamic action of two temperate New Zealand finfish Chrysophrys auratus and Aldrichetta forsteri. J Comp Physiol B 190, 169–183 (2020). https://doi.org/10.1007/s00360-020-01258-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-020-01258-5