Abstract
Objectives
To perform a systematic review regarding the developments in the field of radiomics in lymphoma. To evaluate the quality of included articles by the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2), the phases classification criteria for image mining studies, and the radiomics quality scoring (RQS) tool.
Methods
We searched for eligible articles in the MEDLINE/PubMed and EMBASE databases using the terms “radiomics”, “texture” and “lymphoma”. The included studies were divided into two categories: diagnosis-, therapy response- and outcome-related studies. The diagnosis-related studies were evaluated using the QUADAS-2; all studies were evaluated using the phases classification criteria for image mining studies and the RQS tool by two reviewers.
Results
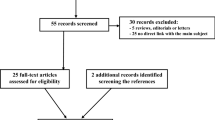
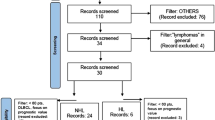
Forty-five studies were included; thirteen papers (28.9%) focused on the differential diagnosis of primary central nervous system lymphoma (PCNSL) and glioblastoma (GBM). Thirty-two (71.1%) studies were classified as discovery science according to the phase classification criteria for image mining studies. The mean RQS score of all studies was 14.2% (ranging from 0.0 to 40.3%), and 23 studies (51.1%) were given a score of < 10%.
Conclusion
The radiomics features could serve as diagnostic and prognostic indicators in lymphoma. However, the current conclusions should be interpreted with caution due to the suboptimal quality of the studies. In order to introduce radiomics into lymphoma clinical settings, the lesion segmentation and selection, the influence of the pathological pattern and the extraction of multiple modalities and multiple time points features need to be further studied.
Key Points
• The radiomics approach may provide useful information for diagnosis, prediction of the therapy response, and outcome of lymphoma.
• The quality of published radiomics studies in lymphoma has been suboptimal to date.
• More studies are needed to examine lesion selection and segmentation, the influence of pathological patterns, and the extraction of multiple modalities and multiple time point features.



Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the curve
- CT:
-
Computed tomography
- DLBCL:
-
Diffuse large B cell lymphoma
- GBM:
-
Glioblastoma
- HL:
-
Hodgkin’s lymphoma
- MRI:
-
Magnetic resonance imaging
- NHL:
-
Non-Hodgkin’s lymphoma
- PCNSL:
-
Primary central nervous system lymphoma
- PET:
-
Positron emission tomography
- QUADAS-2:
-
The Quality Assessment of Diagnostic Accuracy Studies-2
- RQS:
-
Radiomics quality scoring
References
Lambin P, Rios-Velazquez E, Leijenaar R et al (2012) Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer 48:441–446
Patyk M, Silicki J, Mazur R, Kręcichwost R, Sokołowska-Dąbek D, Zaleska-Dorobisz U (2018) Radiomics – the value of the numbers in present and future radiology. Pol J Radiol 83:e171–e174
Acharya UR, Hagiwara Y, Sudarshan VK, Chan WY, Ng KH (2018) Towards precision medicine: from quantitative imaging to radiomics. J Zhejiang Univ Sci B 19:6–24
Hatt M, Tixier F, Pierce L, Kinahan PE, Le Rest CC, Visvikis D (2017) Characterization of PET/CT images using texture analysis: the past, the present… any future? Eur J Nucl Med Mol Imaging 44:151–165
McGranahan N, Swanton C (2017) Clonal heterogeneity and tumor evolution: past, present, and the future. Cell 168:613–628
Schwarz RF, Ng CKY, Cooke SL et al (2015) Spatial and temporal heterogeneity in high-grade serous ovarian cancer: a phylogenetic analysis. PLoS Med 12:e1001789
Wilson R, Devaraj A (2017) Radiomics of pulmonary nodules and lung cancer. Transl Lung Cancer Res 6:86–91
Tandel GS, Biswas M, Kakde OG et al (2019) A review on a deep learning perspective in brain cancer classification. Cancers (Basel) 11
Wong AJ, Kanwar A, Mohamed AS, CD Fuller (2016) Radiomics in head and neck cancer: from exploration to application. Transl Cancer Res 5:371–382
Jeong WK, Jamshidi N, Felker ER, SS Raman, Lu DS (2019) Radiomics and radiogenomics of primary liver cancers. Clin Mol Hepatol 25:21–29
Horvat N, Bates DDB, Petkovska I (2019) Novel imaging techniques of rectal cancer: what do radiomics and radiogenomics have to offer? A literature review. Abdom Radiol (NY) 44:3764–3774
Swerdlow SH, Campo E, Pileri SA et al (2016) The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 127:2375–2390
Schürch CM, Federmann B, Quintanilla-Martinez L, Fend F (2018) Tumor heterogeneity in lymphomas: a different breed. Pathobiology 85:130–145
Lambin P, Leijenaar RTH, Deist TM et al (2017) Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 14:749–762
Sollini M, Antunovic L, Chiti A, Kirienko M (2019) Towards clinical application of image mining: a systematic review on artificial intelligence and radiomics. Eur J Nucl Med Mol Imaging 46:2656–2672
Zorzela L, Loke YK, Ioannidis JP et al (2016) PRISMA harms checklist: improving harms reporting in systematic reviews. BMJ 352:i157
Whiting PF, Rutjes AWS, Westwood ME et al (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155:529–536
Sheskin D (2007) Handbook of parametric and nonparametric statistical procedures, 4th edn. Chapman & Hall/CRC, Web, Boca Raton
Joseph LF, Bruce L, Myunghee Cho P (2004) Wiley series in probability and statistics. John Wiley & Sons, Web, Hoboken
Bathla G, Soni N, Endozo R, Ganeshan B (2019) Magnetic resonance texture analysis utility in differentiating intraparenchymal neurosarcoidosis from primary central nervous system lymphoma: a preliminary analysis. Neuroradiol J 32:203–209
Wang B, Liu M, Chen Z (2019) Differential diagnostic value of texture feature analysis of magnetic resonance T2 weighted imaging between glioblastoma and primary central neural system lymphoma. Chin Med Sci J 34:10–17
Wu G, Chen Y, Wang Y et al (2018) Sparse representation-based radiomics for the diagnosis of brain tumors. IEEE Trans Med Imaging 37:893–905
Yun J, Park JE, Lee H, Ham S, Kim N, Kim HS (2019) Radiomic features and multilayer perceptron network classifier: a robust MRI classification strategy for distinguishing glioblastoma from primary central nervous system lymphoma. Sci Rep 9:5746
Kunimatsu A, Kunimatsu N, Yasaka K et al (2019) Machine learning-based texture analysis of contrast-enhanced mr imaging to differentiate between glioblastoma and primary central nervous system lymphoma. Magn Reson Med Sci 18:44–52
Kunimatsu A, Kunimatsu N, Kamiya K, Watadani T, Mori H, Abe O (2018) Comparison between glioblastoma and primary central nervous system lymphoma using MR image-based texture analysis. Magn Reson Med Sci 17:50–57
Kim Y, Cho H h, Kim ST, Park H, Nam D, Kong DS (2018) Radiomics features to distinguish glioblastoma from primary central nervous system lymphoma on multi-parametric MRI. Neuroradiology 60:1297–1305
Suh HB, Choi YS, Bae S et al (2018) Primary central nervous system lymphoma and atypical glioblastoma: differentiation using radiomics approach. Eur Radiol 28:3832–3839
Kang D, Park JE, Kim YH et al (2018) Diffusion radiomics as a diagnostic modal for atypical manifestation of primary central nervous system lymphoma: development and multicenter external validation. Neuro Oncol 20:1251–1261
Nakagawa M, Nakaura T, Namimoto T et al (2018) Machine learning based on multi-parametric magnetic resonance imaging to differentiate glioblastoma multiforme from primary cerebral nervous system lymphoma. Eur J Radiol 108:147–154
Xiao DD, Yan PF, Wang YX, Osman MS, Zhao HY (2018) Glioblastoma and primary central nervous system lymphoma: preoperative differentiation by using MRI-based 3D texture analysis. Clin Neurol Neurosurg 173:84–90
Chen Y, Li Z, Wu G et al (2018) Primary central nervous system lymphoma and glioblastoma differentiation based on conventional magnetic resonance imaging by high-throughput SIFT features. Int J Neurosci 128:608–618
Alcaide-Leon P, Dufort P, Geraldo AF et al (2017) Differentiation of enhancing glioma and primary central nervous system lymphoma by texture-based machine learning. AJNR Am J Neuroradiol 38:1145–1150
Guo J, Liu Z, Shen C et al (2018) MR-based radiomics signature in differentiating ocular adnexal lymphoma from idiopathic orbital inflammation. Eur Radiol 28:3872–3881
Fujima N, Homma A, Harada T et al (2019) The utility of MRI histogram and texture analysis for the prediction of histological diagnosis in head and neck malignancies. Cancer Imaging 19:5
Wu X, Sikiö M, Pertovaara H et al (2016) Differentiation of diffuse large B-cell lymphoma from follicular lymphoma using texture analysis on conventional MR images at 3.0 tesla. Acad Radiol 23:696–703
Ba-Ssalamah A, Muin D, Schernthaner R et al (2013) Texture-based classification of different gastric tumors at contrast-enhanced CT. Eur J Radiol 82:e537–e543
Ma Z, Fang M, Huang Y et al (2017) CT-based radiomics signature for differentiating Borrmann type IV gastric cancer from primary gastric lymphoma. Eur J Radiol 91:142–147
Huang Z, Li M, He D et al (2019) Two-dimensional texture analysis based on CT images to differentiate pancreatic lymphoma and pancreatic adenocarcinoma: a preliminary study. Acad Radiol 26:e189–e195
Reinert CP, Federmann B, Hofmann J et al (2019) Computed tomography textural analysis for the differentiation of chronic lymphocytic leukemia and diffuse large B cell lymphoma of Richter syndrome. Eur Radiol 29:6911–6921
Seidler M, Forghani B, Reinhold C et al (2019) Dual-energy CT texture analysis with machine learning for the evaluation and characterization of cervical lymphadenopathy. Comput Struct Biotechnol J 17:1009–1015
Reinert CP, Kloth C, Fritz J, Nikolaou K, Horger M (2018) Discriminatory CT-textural features in splenic infiltration of lymphoma versus splenomegaly in liver cirrhosis versus normal spleens in controls and evaluation of their role for longitudinal lymphoma monitoring. Eur J Radiol 104:129–135
Kong Z, Jiang C, Zhu R et al (2019) 18F-FDG-PET-based radiomics features to distinguish primary central nervous system lymphoma from glioblastoma. Neuroimage Clin 23:101912
Aide N, Talbot M, Fruchart C, Damaj G, Lasnon C (2018) Diagnostic and prognostic value of baseline FDG PET/CT skeletal textural features in diffuse large B cell lymphoma. Eur J Nucl Med Mol Imaging 45:699–711
Lippi M, Gianotti S, Fama A et al (2019) Texture analysis and multiple-instance learning for the classification of malignant lymphomas. Comput Methods Prog Biomed 185:105153
Xu H, Guo W, Cui X et al (2019) Three-dimensional texture analysis based on PET/CT images to distinguish hepatocellular carcinoma and hepatic lymphoma. Front Oncol 9:844
Zhu S, Xu H, Shen C et al (2019) Differential diagnostic ability of 18F-FDG PET/CT radiomics features between renal cell carcinoma and renal lymphoma. Q J Nucl Med Mol Imaging. https://doi.org/10.23736/S1824-4785.19.03137-6
Ou X, Wang J, Zhou R et al (2019) Ability of 18 F-FDG PET / CT radiomic features to distinguish breast carcinoma from breast lymphoma. Contrast Media Mol Imaging 2019:4507694
Ou X, Zhang J, Wang J et al (2019) Radiomics based on 18F-FDG PET/CT could differentiate breast carcinoma from breast lymphoma using machine-learning approach: a preliminary study. Cancer Med 9:496–506
Lartizien C, Rogez M, Niaf E, Ricard F (2014) Computer-aided staging of lymphoma patients with FDG PET/CT imaging based on textural information. IEEE J Biomed Health Inform 18:946–955
Harrison LC, Luukkaala T, Pertovaara H et al (2009) Non-Hodgkin lymphoma response evaluation with MRI texture classification. J Exp Clin Cancer Res 28:87
Harrison L, Dastidar P, Eskola H et al (2008) Texture analysis on MRI images of non-Hodgkin lymphoma. Comput Biol Med 38:519–524
Chen C, Zhuo H, Wei X, Ma X (2019) Contrast-enhanced MRI texture parameters as potential prognostic factors for primary central nervous system lymphoma patients receiving high-dose methotrexate-based chemotherapy. Contrast Media Mol Imaging 2019:5481491
Ganeshan B, Miles KA, Babikir S et al (2017) CT-based texture analysis potentially provides prognostic information complementary to interim FDG-pet for patients with Hodgkin’s and aggressive non-Hodgkin’s lymphomas. Eur Radiol 27:1012–1020
Knogler T, El-Rabadi K, Weber M, Karanikas G, Mayerhoefer ME (2014) Three-dimensional texture analysis of contrast enhanced CT images for treatment response assessment in Hodgkin lymphoma: comparison with F-18-FDG PET. Med Phys 41:121904
Wang M, Xu H, Xiao L, Song W, Zhu S, Ma X (2019) Prognostic value of functional parameters of 18 F-FDG-PET images in patients with primary renal/adrenal lymphoma. Contrast Media Mol Imaging 2019:2641627
Mayerhoefer ME, Riedl CC, Kumar A et al (2019) Radiomic features of glucose metabolism enable prediction of outcome in mantle cell lymphoma. Eur J Nucl Med Mol Imaging 46:2760–2769
Parvez A, Tau N, Hussey D, Maganti M, Metser U (2018) 18F-FDG PET/CT metabolic tumor parameters and radiomics features in aggressive non-Hodgkin’s lymphoma as predictors of treatment outcome and survival. Ann Nucl Med 32:410–416
Milgrom SA, Elhalawani H, Lee J et al (2019) A PET radiomics model to predict refractory mediastinal Hodgkin lymphoma. Sci Rep 9:1322
Ben Bouallègue F, Tabaa YA, Kafrouni M, Cartron G, Vauchot F, Mariano-Goulart D (2017) Association between textural and morphological tumor indices on baseline PET-CT and early metabolic response on interim PET-CT in bulky malignant lymphomas. Med Phys 44:4608–4619
Wu J, Lian C, Ruan S et al (2018) Treatment outcome prediction for cancer patients based on radiomics and belief function theory. IEEE Trans Radiat Plasma Med Sci 3:216–224
Tatsumi M, Isohashi K, Matsunaga K et al (2019) Volumetric and texture analysis on FDG PET in evaluating and predicting treatment response and recurrence after chemotherapy in follicular lymphoma. Int J Clin Oncol 24:1292–1300
Lue KH, Wu YF, Liu SH et al (2019) Prognostic value of pretreatment radiomic features of 18F-FDG PET in patients with Hodgkin lymphoma. Clin Nucl Med 44:e559–e565
Lue KH, Wu YF, Liu SH et al (2019) Intratumor heterogeneity assessed by 18F-FDG PET/CT predicts treatment response and survival outcomes in patients with Hodgkin lymphoma. Acad Radiol. https://doi.org/10.1016/j.acra.2019.10.015
Zhou Y, Ma XL, Pu LT, Zhou RF, Ou XJ, Tian R (2019) Prediction of overall survival and progression-free survival by the 18F-FDG PET/CT radiomic features in patients with primary gastric diffuse large B-cell lymphoma. Contrast Media Mol Imaging 2019:5963607
Chalkidou A, O’Doherty MJ, Marsden PK (2015) False discovery rates in PET and CT studies with texture features: a systematic review. PLoS One 10:e0124165
Forghani R, Savadjiev P, Chatterjee A, Muthukrishnan N, Reinhold C, Forghani B (2019) Radiomics and artificial intelligence for biomarker and prediction model development in oncology. Comput Struct Biotechnol J 17:995–1008
Sanduleanu S, Woodruff HC, de Jong EEC et al (2018) Tracking tumor biology with radiomics: a systematic review utilizing a radiomics quality score. Radiother Oncol 127:349–360
Park JE, Kim D, Kim HS et al (2019) Quality of science and reporting of radiomics in oncologic studies: room for improvement according to radiomics quality score and TRIPOD statement. Eur Radiol 30:523–536
Liu Z, Wang S, Dong D et al (2019) The applications of radiomics in precision diagnosis and treatment of oncology: opportunities and challenges. Theranostics 9:1303–1322
Kalpathy-Cramer J, Freymann JB, Kirby JS, , Kinahan PE, Prior FW (2014) Quantitative imaging network: data sharing and competitive algorithmvalidation leveraging the cancer imaging archive. Transl Oncol 7:147–152
Collins GS, Reitsma JB, Altman DG, Moons KGM, (2015) Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ 350:g7594
Pavic M, Bogowicz M, Würms X et al (2018) Influence of inter-observer delineation variability on radiomics stability in different tumor sites. Acta Oncol 57:1070–1074
Geets X, Lee JA, Bol A, Lonneux M, Grégoire V (2007) A gradient-based method for segmenting FDG-PET images: methodology and validation. Eur J Nucl Med Mol Imaging 34:1427–1438
Hatt M, Cheze le Rest C, Descourt P et al (2010) Accurate automatic delineation of heterogeneous functional volumes in positron emission tomography for oncology applications. Int J Radiat Oncol Biol Phys 77:301–308
Orlhac F, Soussan M, Chouahnia K, Martinod E, Buvat I (2015) 18F-FDG PET-derived textural indices reflect tissue-specific uptake pattern in non-small cell lung cancer. PLoS One 10:e0145063
Brooks FJ, Grigsby PW (2014) The effect of small tumor volumes on studies of intratumoral heterogeneity of tracer uptake. J Nucl Med 55:37–42
Hatt M, Majdoub M, Vallieres M et al (2015) 18F-FDG PET uptake characterization through texture analysis: investigating the complementary nature of heterogeneity and functional tumor volume in a multi-cancer site patient cohort. J Nucl Med 56:38–44
Ha S, Choi H, Paeng JC et al (2019) Radiomics in oncological PET/CT: a methodological overview. Nucl Med Mol Imaging 53:14–29
Wang H, Shen G, Jiang C, Li L, Cui F, Tian R (2018) Prognostic value of baseline, interim and end-of-treatment18F-FDG PET/CT parameters in extranodal natural killer/T-cell lymphoma: a meta-analysis. PLoS One 13:e0194435
Sollini M, Cozzi L, Ninatti G et al (2020) PET/CT radiomics in breast cancer: mind the step. Methods. https://doi.org/10.1016/j.ymeth.2020.01.007
Funding
This study has received funding from the Key Projects of the Ministry of Science and Technology (grant 2017YFC0113304).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Rong Tian, PhD.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was not required for this study because it is a systematic review.
Ethical approval
Institutional review board approval was not required because it is a systematic review.
Methodology
• Retrospective
• Diagnostic or prognostic study
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 72 kb)
Rights and permissions
About this article
Cite this article
Wang, H., Zhou, Y., Li, L. et al. Current status and quality of radiomics studies in lymphoma: a systematic review. Eur Radiol 30, 6228–6240 (2020). https://doi.org/10.1007/s00330-020-06927-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-06927-1