Abstract
Objectives
To evaluate MRI texture analysis in differentiating clinicopathological characteristics of cervical carcinoma (CC).
Methods
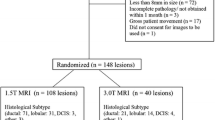
Patients with newly diagnosed CC who underwent pre-treatment MRI were retrospectively reviewed. Texture analysis was performed using commercial software (TexRAD). Largest single-slice ROIs were manually drawn around the tumour on T2-weighted (T2W) images, apparent diffusion coefficient (ADC) maps and contrast-enhanced T1-weighted (T1c) images. First-order texture features were calculated and compared among histological subtypes, tumour grades, FIGO stages and nodal status using the Mann-Whitney U test. Feature selection was achieved by elastic net. Selected features from different sequences were used to build the multivariable support vector machine (SVM) models and the performances were assessed by ROC curves and AUC.
Results
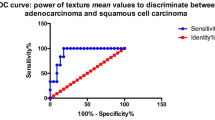
Ninety-five patients with FIGO stage IB~IVB were evaluated. A number of texture features from multiple sequences were significantly different among all the clinicopathological subgroups (p < 0.05). Texture features from different sequences were selected to build the SVM models. The AUCs of SVM models for discriminating histological subtypes, tumour grades, FIGO stages and nodal status were 0.841, 0.850, 0.898 and 0.879, respectively.
Conclusions
Texture features derived from multiple sequences were helpful in differentiating the clinicopathological signatures of CC. The SVM models with selected features from different sequences offered excellent diagnostic discrimination of the tumour characteristics in CC.
Key Points
• First-order texture features are able to differentiate clinicopathological signatures of cervical carcinoma.
• Combined texture features from different sequences can offer excellent diagnostic discrimination of the tumour characteristics in cervical carcinoma.

Similar content being viewed by others
Abbreviations
- ACA:
-
Adenocarcinoma
- ADC:
-
Apparent diffusion coefficient
- AUC:
-
Area under the curve
- CC:
-
Cervical carcinoma
- DWI:
-
Diffusion-weighted imaging
- MPP:
-
Mean of positive pixels
- MRI:
-
Magnetic resonance imaging
- ROI:
-
Region of interest
- SCC:
-
Squamous cell carcinoma
- SD:
-
Standard deviation
- SSF:
-
Spatial scale filter
- SVM:
-
Support vector machine
- T1c:
-
Contrast-enhanced T1-weighted
- T2W:
-
T2-weighted
- VOI:
-
Volume of interest
References
Koh DM, Collins DJ (2007) Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol 188(6):1622–1635
Exner M, Kuhn A, Stumpp P et al (2016) Value of diffusion-weighted MRI in diagnosis of uterine cervical cancer: a prospective study evaluating the benefits of DWI compared to conventional MR sequences in a 3T environment. Acta Radiol 57(7):869–877
Kuang F, Ren J, Zhong Q, Liyuan F, Huan Y, Chen Z (2013) The value of apparent diffusion coefficient in the assessment of cervical cancer. Eur Radiol 23(4):1050–1058
Karunya RJ, Tharani P, John S, Kumar RM, Das S (2017) Role of functional magnetic resonance imaging derived parameters as imaging biomarkers and correlation with clinicopathological features in carcinoma of uterine cervix. J Clin Diagn Res 11(8):Xc06-xc11
Liu Y, Ye Z, Sun H, Bai R (2015) Clinical application of diffusion-weighted magnetic resonance imaging in uterine cervical cancer. Int J Gynecol Cancer 25(6):1073–1078
Liu Y, Ye Z, Sun H, Bai R (2013) Grading of uterine cervical cancer by using the ADC difference value and its correlation with microvascular density and vascular endothelial growth factor. Eur Radiol 23(3):757–765
Akita A, Shinmoto H, Hayashi S et al (2011) Comparison of T2-weighted and contrast-enhanced T1-weighted MR imaging at 1.5 T for assessing the local extent of cervical carcinoma. Eur Radiol 21(9):1850–1857
Davnall F, Yip CS, Ljungqvist G et al (2012) Assessment of tumor heterogeneity: an emerging imaging tool for clinical practice? Insights Imaging 3(6):573–589
Meng J, Liu S, Zhu L et al (2018) Texture analysis as imaging biomarker for recurrence in advanced cervical cancer treated with CCRT. Sci Rep 8(1):11399
Ciolina M, Vinci V, Villani L et al (2019) Texture analysis versus conventional MRI prognostic factors in predicting tumor response to neoadjuvant chemotherapy in patients with locally advanced cancer of the uterine cervix. Radiol Med 124(10):955–964
Meng J, Zhu L, Zhu L et al (2017) Whole-lesion ADC histogram and texture analysis in predicting recurrence of cervical cancer treated with CCRT. Oncotarget 8(54):92442–92453
Guan Y, Li W, Jiang Z et al (2017) Value of whole-lesion apparent diffusion coefficient (ADC) first-order statistics and texture features in clinical staging of cervical cancers. Clin Radiol 72(11):951–958
Becker AS, Ghafoor S, Marcon M et al (2017) MRI texture features may predict differentiation and nodal stage of cervical cancer: a pilot study. Acta Radiol Open 6(10):2058460117729574
Liu Y, Zhang Y, Cheng R et al (2019) Radiomics analysis of apparent diffusion coefficient in cervical cancer: a preliminary study on histological grade evaluation. J Magn Reson Imaging 49(1):280–290
Kurman RJ, Carcangiu ML, Herrington CS, Young RH (2014) WHO classification of tumours of female reproductive organs. International Agency for Research on Cancer, Lyon
Bhatla N, Berek JS, Cuello Fredes M et al (2019) Revised FIGO staging for carcinoma of the cervix uteri. Int J Gynaecol Obstet 145(1):129–135
Choi HJ, Kim SH, Seo SS et al (2006) MRI for pretreatment lymph node staging in uterine cervical cancer. AJR Am J Roentgenol 187(5):W538–W543
Kim SH, Kim SC, Choi BI, Han MC (1994) Uterine cervical carcinoma: evaluation of pelvic lymph node metastasis with MR imaging. Radiology 190(3):807–811
Balleyguier C, Sala E, Da Cunha T et al (2011) Staging of uterine cervical cancer with MRI: guidelines of the European Society of Urogenital Radiology. Eur Radiol 21(5):1102–1110
Miles KA, Ganeshan B, Hayball MP (2013) CT texture analysis using the filtration-histogram method: what do the measurements mean? Cancer Imaging 13(3):400–406
Zou H, Hastie T (2005) Regularization and variable selection via the elastic net. J R Statist Soc B 67(2):301–320
Parmar C, Grossmann P, Bussink J, Lambin P, Aerts H (2015) Machine learning methods for quantitative radiomic biomarkers. Sci Rep 5:13087
Mandrekar JN (2010) Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol 5(9):1315–1316
Mu W, Chen Z, Liang Y et al (2015) Staging of cervical cancer based on tumor heterogeneity characterized by texture features on (18)F-FDG PET images. Phys Med Biol 60(13):5123–5139
Torheim T, Malinen E, Kvaal K et al (2014) Classification of dynamic contrast enhanced MR images of cervical cancers using texture analysis and support vector machines. IEEE Trans Med Imaging 33(8):1648–1656
Lucia F, Visvikis D, Desseroit MC (2018) Prediction of outcome using pretreatment (18)F-FDG PET/CT and MRI radiomics in locally advanced cervical cancer treated with chemoradiotherapy. Eur J Nucl Med Mol Imaging 45(5):768–786
Ueno Y, Forghani B, Forghani R et al (2017) Endometrial carcinoma: MR imaging-based texture model for preoperative risk stratification-a preliminary analysis. Radiology 284(3):748–757
Ytre-Hauge S, Dybvik JA, Lundervold A et al (2018) Preoperative tumor texture analysis on MRI predicts high-risk disease and reduced survival in endometrial cancer. J Magn Reson Imaging 48(6):1637–1647
Hameed M, Ganeshan B, Shur J, Mukherjee S, Afaq A, Batura D (2019) The clinical utility of prostate cancer heterogeneity using texture analysis of multiparametric MRI. Int Urol Nephrol 51(5):817–824
Goyal A, Razik A, Kandasamy D et al (2019) Role of MR texture analysis in histological subtyping and grading of renal cell carcinoma: a preliminary study. Abdom Radiol (NY) 44(10):3336–3349
Gourtsoyianni S, Doumou G, Prezzi D et al (2017) Primary rectal cancer: repeatability of global and local-regional MR imaging texture features. Radiology 284(2):552–561
Wang M, Perucho JAU, Chan Q et al (2020) Diffusion kurtosis imaging in the assessment of cervical carcinoma. Acad Radiol 27(5):e94–e101
McCluggage WG (2018) Towards developing a meaningful grading system for cervical squamous cell carcinoma. J Pathol Clin Res 4(2):81–85
Noviello MB, Silva-Filho AL, Traiman P et al (2008) Inter- and intraobserver variability in the assessment of tumor grade and lymphovascular space invasion in patients with squamous cell carcinoma of the cervix. Eur J Obstet Gynecol Reprod Biol 138(2):246–248
Yang F, Young L, Grigsby P (2016) Predictive value of standardized intratumoral metabolic heterogeneity in locally advanced cervical cancer treated with chemoradiation. Int J Gynecol Cancer 26(4):777–784
Dercle L, Ammari S, Bateson M et al (2017) Limits of radiomic-based entropy as a surrogate of tumor heterogeneity: ROI-area, acquisition protocol and tissue site exert substantial influence. Sci Rep 7(1):7952
Jalil O, Afaq A, Ganeshan B et al (2017) Magnetic resonance based texture parameters as potential imaging biomarkers for predicting long-term survival in locally advanced rectal cancer treated by chemoradiotherapy. Colorectal Dis 19(4):349–362
Liu Z, Wang S, Dong D et al (2019) The applications of radiomics in precision diagnosis and treatment of oncology: opportunities and challenges. Theranostics 9(5):1303–1322
Napel S, Mu W, Jardim-Perassi BV, Aerts H, Gillies RJ (2018) Quantitative imaging of cancer in the postgenomic era: radio (geno) mics, deep learning, and habitats. Cancer 124(24):4633–4649
Wang T, Gao T, Guo H et al (2020) Preoperative prediction of parametrial invasion in early-stage cervical cancer with MRI-based radiomics nomogram. Eur Radiol. https://doi.org/10.1007/s00330-019-06655-1
Wang T, Gao T, Yang J et al (2019) Preoperative prediction of pelvic lymph nodes metastasis in early-stage cervical cancer using radiomics nomogram developed based on T2-weighted MRI and diffusion-weighted imaging. Eur J Radiol 114:128–135
De Cecco CN, Ganeshan B, Ciolina M et al (2015) Texture analysis as imaging biomarker of tumoral response to neoadjuvant chemoradiotherapy in rectal cancer patients studied with 3-T magnetic resonance. Invest Radiol 50(4):239–245
Funding
This study has received funding from the General Research Fund (GRF, No. 17119916) of the Research Grants Council (RGC), Hong Kong.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Elaine Y.P. Lee.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board (Reference No. UW 17-389) approval was obtained.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported in Academic Radiology that evaluated the clinical value of diffusion kurtosis imaging and not assessed texture analysis.
Methodology
• Retrospective
• Observational
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, M., Perucho, J.A.U., Tse, K.Y. et al. MRI texture features differentiate clinicopathological characteristics of cervical carcinoma. Eur Radiol 30, 5384–5391 (2020). https://doi.org/10.1007/s00330-020-06913-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-06913-7