Abstract
Objectives
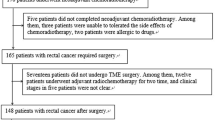
This study aimed to evaluate the efficiency of imaging features and texture analysis (TA) based on baseline rectal MRI for the early prediction of therapeutic response to neoadjuvant chemoradiotherapy (nCRT) and tumor recurrence in patients with locally advanced rectal cancer (LARC).
Methods
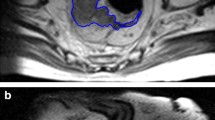
Consecutive patients with LARC who underwent rectal MRI between January 2014 and December 2015 and surgical resection after completing nCRT were retrospectively enrolled. Imaging features were analyzed, and TA parameters were extracted from the tumor volume of interest (VOI) from baseline rectal MRI. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the optimal TA parameter cutoff values to stratify the patients. Logistic and Cox regression analyses were performed to assess the efficacy of each imaging feature and texture parameter in predicting tumor response and disease-free survival.
Results
In total, 78 consecutive patients were enrolled. In the logistic regression, good treatment response was associated with lower tumor location (OR = 13.284, p = 0.012), low Conv_Min (OR = 0.300, p = 0.013) and high Conv_Std (OR = 3.174, p = 0.016), Shape_Sphericity (OR = 3.170, p = 0.015), and Shape_Compacity (OR = 2.779, p = 0.032). In the Cox regression, a greater risk of tumor recurrence was related to higher cT stage (HR = 5.374, p = 0.044), pelvic side wall lymph node positivity (HR = 2.721, p = 0.013), and gray-level run length matrix_long-run low gray-level emphasis (HR = 2.268, p = 0.046).
Conclusions
Imaging features and TA based on baseline rectal MRI could be valuable for predicting the treatment response to nCRT for rectal cancer and tumor recurrence.
Key Points
• Imaging features and texture parameters of T2-weighted MR images of rectal cancer can help to predict treatment response and the risk for tumor recurrence.
• Tumor location as well as conventional and shape indices of texture features can help to predict treatment response for rectal cancer.
• Clinical T stage, positive pelvic side wall lymph nodes, and the high-order texture parameter, GLRLM_LRLGE, can help to predict tumor recurrence for rectal cancer.


Similar content being viewed by others
Abbreviations
- 18FDG-PET:
-
Fluorine-18 fluorodeoxyglucose positron emission tomography
- ADC:
-
Apparent diffusion coefficient
- CI:
-
Confidence interval
- cN:
-
Clinical N
- cT:
-
Clinical T
- DCE:
-
Dynamic contrast-enhanced
- DFS:
-
Disease-free survival
- DWI:
-
Diffusion-weighted echo-planar imaging
- EMVI:
-
Extramural venous invasion
- GLCM:
-
Gray-level co-occurrence matrix
- GLRLM:
-
Gray-level run length matrix
- GLRLM_LRLGE:
-
Gray-level run length matrix_long-run low gray-level emphasis
- GLZLM:
-
Gray-level zone length matrix
- HR:
-
Hazard ratio
- LARC:
-
Locally advanced rectal cancer
- LN:
-
Lymph node
- MRF:
-
Mesorectal fascia
- MRI:
-
Magnetic resonance imaging
- N+:
-
Lymph node metastasis positive
- nCRT:
-
Neoadjuvant chemoradiotherapy
- NGLDM:
-
Neighborhood gray-level different matrix
- OR:
-
Odds ratio
- pCR:
-
Pathologic complete response
- PD:
-
Progressive disease
- ROC:
-
Receiver operating characteristic
- T2WI:
-
T2-weighted images
- TA:
-
Texture analysis
- TRG:
-
Tumor regression grade
- VOI:
-
Volume of interest
References
Cui Y, Yang X, Shi Z et al (2019) Radiomics analysis of multiparametric MRI for prediction of pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Eur Radiol 29:1211–1220
Ryan JE, Warrier SK, Lynch AC, Ramsay RG, Phillips WA, Heriot AG (2016) Predicting pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer: a systematic review. Colorectal Dis 18:234–246
Meng Y, Zhang C, Zou S et al (2018) MRI texture analysis in predicting treatment response to neoadjuvant chemoradiotherapy in rectal cancer. Oncotarget 9:11999
Prezzi D, Goh V (2016) Rectal cancer magnetic resonance imaging: imaging beyond morphology. Clin Oncol (R Coll Radiol) 28:83–92
Lambregts DMJ, Maas M, Boellaard TN et al (2020) Long-term imaging characteristics of clinical complete responders during watch-and-wait for rectal cancer—an evaluation of over 1500 MRIs. Eur Radiol 30:272–280
Lambregts DM, Boellaard TN, Beets-Tan RG (2019) Response evaluation after neoadjuvant treatment for rectal cancer using modern MR imaging: a pictorial review. Insights Imaging 10:15
Santiago I, Barata M, Figueiredo N et al (2020) The split scar sign as an indicator of sustained complete response after neoadjuvant therapy in rectal cancer. Eur Radiol 30:224–238
Hotker AM, Garcia-Aguilar J, Gollub MJ (2014) Multi-parametric MRI of rectal cancer in the assessment of response to therapy: a systematic review. Dis Colon Rectum 57:790
Joye I, Deroose CM, Vandecaveye V, Haustermans K (2014) The role of diffusion-weighted MRI and (18)F-FDG PET/CT in the prediction of pathologic complete response after radiochemotherapy for rectal cancer: a systematic review. Radiother Oncol 113:158–165
Liu Z, Wang S, Dong D et al (2019) The applications of radiomics in precision diagnosis and treatment of oncology: opportunities and challenges. Theranostics 9:1303–1322
Lubner MG, Smith AD, Sandrasegaran K, Sahani DV, Pickhardt PJ (2017) CT texture analysis: definitions, applications, biologic correlates, and challenges. Radiographics 37:1483–1503
Jalil O, Afaq A, Ganeshan B et al (2017) Magnetic resonance based texture parameters as potential imaging biomarkers for predicting long-term survival in locally advanced rectal cancer treated by chemoradiotherapy. Colorectal Dis 19:349–362
Nardone V, Reginelli A, Scala F et al (2019) Magnetic-resonance-imaging texture analysis predicts early progression in rectal cancer patients undergoing neoadjuvant chemoradiation. Gastroenterol Res Pract 2019:8505798
Horvat N, Veeraraghavan H, Khan M et al (2018) MR imaging of rectal cancer: radiomics analysis to assess treatment response after neoadjuvant therapy. Radiology 287:833–843
Shu Z, Fang S, Ye Q et al (2019) Prediction of efficacy of neoadjuvant chemoradiotherapy for rectal cancer: the value of texture analysis of magnetic resonance images. Abdom Radiol (NY) 44:3775–3784
Vandendorpe B, Durot C, Lebellec L et al (2019) Prognostic value of the texture analysis parameters of the initial computed tomographic scan for response to neoadjuvant chemoradiation therapy in patients with locally advanced rectal cancer. Radiother Oncol 135:153–160
Liu Z, Zhang X-Y, Shi Y-J et al (2017) Radiomics analysis for evaluation of pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Clin Cancer Res 23:7253–7262
Grossmann P, Narayan V, Chang K et al (2017) Quantitative imaging biomarkers for risk stratification of patients with recurrent glioblastoma treated with bevacizumab. Neuro Oncol 19:1688–1697
Yang L, Qiu M, Xia C et al (2019) Value of high-resolution DWI in combination with texture analysis for the evaluation of tumor response after preoperative chemoradiotherapy for locally advanced rectal cancer. AJR Am J Roentgenol:1–8. https://doi.org/10.2214/AJR.18.20689
Ahmed A, Gibbs P, Pickles M, Turnbull L (2013) Texture analysis in assessment and prediction of chemotherapy response in breast cancer. J Magn Reson Imaging 38:89–101
Dworak O, Keilholz L, Hoffmann A (1997) Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis 12:19–23
Liu Y, Xu X, Yin L, Zhang X, Li L, Lu H (2017) Relationship between glioblastoma heterogeneity and survival time: an MR imaging texture analysis. AJNR Am J Neuroradiol 38:1695–1701
Davnall F, Yip CS, Ljungqvist G et al (2012) Assessment of tumor heterogeneity: an emerging imaging tool for clinical practice? Insights Imaging 3:573–589
Dasarathy BV, Holder EB (1991) Image characterizations based on joint gray level—run length distributions. Pattern Recogn Lett 12:497–502
Nielsen B, Albregtsen F, Danielsen HE (2008) Statistical nuclear texture analysis in cancer research: a review of methods and applications. Crit Rev Oncog 14:89–164
Zlobec I, Gunthert U, Tornillo L et al (2009) Systematic assessment of the prognostic impact of membranous CD44v6 protein expression in colorectal cancer. Histopathology 55:564–575
Gourtsoyianni S, Doumou G, Prezzi D et al (2017) Primary rectal cancer: repeatability of global and local-regional MR imaging texture features. Radiology 284:552–561
Miles KA, Ganeshan B, Hayball MP (2013) CT texture analysis using the filtration-histogram method: what do the measurements mean? Cancer Imaging 13:400
Sieren J, Smith A, Thiesse J et al (2011) Exploration of the volumetric composition of human lung cancer nodules in correlated histopathology and computed tomography. Lung Cancer 74:61–68
Hocquelet A, Auriac T, Perier C et al (2018) Pre-treatment magnetic resonance-based texture features as potential imaging biomarkers for predicting event free survival in anal cancer treated by chemoradiotherapy. Eur Radiol 28:2801–2811
De Cecco CN, Ganeshan B, Ciolina M et al (2015) Texture analysis as imaging biomarker of tumoral response to neoadjuvant chemoradiotherapy in rectal cancer patients studied with 3-T magnetic resonance. Invest Radiol 50:239–245
Monguzzi L, Ippolito D, Bernasconi DP, Trattenero C, Galimberti S, Sironi S (2013) Locally advanced rectal cancer: value of ADC mapping in prediction of tumor response to radiochemotherapy. Eur J Radiol 82:234–240
Punt CJ, Buyse M, Kohne CH et al (2007) Endpoints in adjuvant treatment trials: a systematic review of the literature in colon cancer and proposed definitions for future trials. J Natl Cancer Inst 99:998–1003
Beets-Tan RG, Beets GL (2004) Rectal cancer: review with emphasis on MR imaging. Radiology 232:335–346
Zhang H, Hung CL, Min G, Guo JP, Liu M, Hu X (2019) GPU-accelerated GLRLM algorithm for feature extraction of MRI. Sci Rep 9:10883
Varghese BA, Cen SY, Hwang DH, Duddalwar VA (2019) Texture analysis of imaging: what radiologists need to know. AJR Am J Roentgenol 212:520–528
Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: images are more than pictures, they are data. Radiology 278:563–577
Mayerhoefer ME, Szomolanyi P, Jirak D, Materka A, Trattnig S (2009) Effects of MRI acquisition parameter variations and protocol heterogeneity on the results of texture analysis and pattern discrimination: an application-oriented study. Med Phys 36:1236–1243
Funding
The authors declare no funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Kyung Ah Kim.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
Jeongbae Rhie kindly provided statistical advice for this manuscript.
Jeongbae Rhie, one of the authors, has significant statistical expertise.
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was not required for this study because of the retrospective nature of the study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Retrospective
• Diagnostic or prognostic study
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Park, H., Kim, K.A., Jung, JH. et al. MRI features and texture analysis for the early prediction of therapeutic response to neoadjuvant chemoradiotherapy and tumor recurrence of locally advanced rectal cancer. Eur Radiol 30, 4201–4211 (2020). https://doi.org/10.1007/s00330-020-06835-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-06835-4