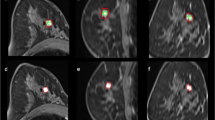
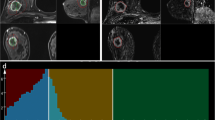
Abstract
Purpose
To investigate the diagnostic capability of whole-lesion (WL) histogram and texture analysis of dynamic contrast-enhanced (DCE) MRI inline-generated quantitative parametric maps using CAIPIRINHA-Dixon-TWIST-VIBE (CDTV) to differentiate malignant from benign breast lesions and breast cancer subtypes.
Materials and methods
From February 2018 to November 2018, DCE MRI using CDTV was performed on 211 patients. The inline-generated parametric maps included Ktrans, kep, Ve, and IAUGC60. Histogram and texture features were extracted from the above parametric maps respectively based on a WL analysis. Student’s t tests, one-way ANOVAs, Mann-Whitney U tests, Jonckheere-Terpstra tests, and ROC curves were used for statistical analysis.
Results
Compared with benign breast lesions, malignant breast lesions showed significantly higher Ktrans_median, 5th percentile, entropy, and diff-entropy, IAUGC60_median, 5th percentile, entropy, and diff-entropy, kep_mean, median, 5th percentile, entropy, and diff-entropy, and Ve_95th percentile, diff-variance, and contrast, and significantly lower kep_skewness and Ve_SD, entropy, diff-entropy, and skewness (all p ≤ 0.011). The combination of all the extracted parameters yielded an AUC of 0.85 (sensitivity 76%, specificity 86%). kep_contrast showed a significant difference among different subtypes of breast cancer (p = 0.006). kep_skewness showed a significant difference between lymph node–positive and lymph node–negative breast cancer (p = 0.007). The IAGC60_5th percentile had an AUC of 0.71 (sensitivity 50%, specificity 91%) for differentiating between high- and low-proliferation groups of breast cancer.
Conclusions
The WL histogram and texture analyses of CDTV-DCE-derived parameters may give additional information for further evaluation of breast cancer.
Key Points
• Inline DCE mapping with CDTV is effective and time-saving.
• WL histogram and texture-extracted features could distinguish breast cancer from benign lesions accurately.
• kep_contrast, kep_skewness, and IAUGC60_5th percentile could predict breast cancer subtypes, lymph node metastasis, and proliferation abilities, respectively.




Similar content being viewed by others
Abbreviations
- AIF:
-
Arterial input function
- AUC:
-
Area under the receiver operating characteristic curve
- BI-RADS:
-
Breast Imaging Reporting and Data System
- CAIPIRINHA:
-
Controlled aliasing in parallel imaging results in higher acceleration
- CDTV:
-
CAIPIRINHA-Dixon-TWIST-VIBE
- DCE:
-
Dynamic contrast enhancement
- ER:
-
Estrogen receptor
- HER2:
-
Human epidermal growth factor receptor-2
- IAUGC60 :
-
Initial area under the gadolinium curve after the first 60 s
- k ep :
-
Outflow rate constant
- K trans :
-
Inflow transfer constant
- PR:
-
Progesterone receptor
- ROC:
-
Receiver operating characteristic
- TE:
-
Echo time
- TR:
-
Reception time
- TWIST:
-
Time-resolved angiography with interleaved stochastic trajectories
- V e :
-
Extravascular extracellular space
- VFA:
-
Variable flip angle
- VIBE:
-
Volumetric interpolated breath-hold examination
- WL:
-
Whole lesion
References
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69:7–34
Peters NH, Borel Rinkes IH, Zuithoff NP, Mali WP, Moons KG, Peeters PH (2008) Meta-analysis of MR imaging in the diagnosis of breast lesions. Radiology 246:116–124
Pickles MD, Lowry M, Manton DJ, Turnbull LW (2015) Prognostic value of DCE-MRI in breast cancer patients undergoing neoadjuvant chemotherapy: a comparison with traditional survival indicators. Eur Radiol 25:1097–1106
Cho N, Im SA, Park IA et al (2014) Breast cancer: early prediction of response to neoadjuvant chemotherapy using parametric response maps for MR imaging. Radiology 272:385–396
Yi B, Kang DK, Yoon D et al (2014) Is there any correlation between model-based perfusion parameters and model-free parameters of time-signal intensity curve on dynamic contrast enhanced MRI in breast cancer patients? Eur Radiol 24:1089–1096
Drisis S, Metens T, Ignatiadis M, Stathopoulos K, Chao SL, Lemort M (2016) Quantitative DCE-MRI for prediction of pathological complete response following neoadjuvant treatment for locally advanced breast cancer: the impact of breast cancer subtypes on the diagnostic accuracy. Eur Radiol 26:1474–1484
Li Z, Ai T, Hu Y et al (2018) Application of whole-lesion histogram analysis of pharmacokinetic parameters in dynamic contrast-enhanced MRI of breast lesions with the CAIPIRINHA-Dixon-TWIST-VIBE technique. J Magn Reson Imaging 47:91–96
Gruber L, Rainer V, Plaikner M, Kremser C, Jaschke W, Henninger B (2018) CAIPIRINHA-Dixon-TWIST (CDT)-VIBE MR imaging of the liver at 3.0T with gadoxetate disodium: a solution for transient arterial-phase respiratory motion-related artifacts? Eur Radiol 28:2013–2021
Qu J, Han S, Zhang H et al (2016) Improved detection of recurrent hepatocellular carcinomas in arterial phase with CAIPIRINHA-Dixon-TWIST-volumetric interpolated breath-hold examination. Invest Radiol 51:602–608
Hao W, Zhao B, Wang G, Wang C, Liu H (2015) Influence of scan duration on the estimation of pharmacokinetic parameters for breast lesions: a study based on CAIPIRINHA-Dixon-TWIST-VIBE technique. Eur Radiol 25:1162–1171
Chen Y, Wu B, Liu H, Wang D, Gu Y (2018) Feasibility study of dual parametric 2D histogram analysis of breast lesions with dynamic contrast-enhanced and diffusion-weighted MRI. J Transl Med 16:325
Kim JH, Ko ES, Lim Y et al (2017) Breast cancer heterogeneity: MR imaging texture analysis and survival outcomes. Radiology 282:665–675
Waugh SA, Purdie CA, Jordan LB et al (2016) Magnetic resonance imaging texture analysis classification of primary breast cancer. Eur Radiol 26:322–330
Henderson S, Purdie C, Michie C et al (2017) Interim heterogeneity changes measured using entropy texture features on T2-weighted MRI at 3.0 T are associated with pathological response to neoadjuvant chemotherapy in primary breast cancer. Eur Radiol 27:4602–4611
Cheng HL, Wright GA (2006) Rapid high-resolution T(1) mapping by variable flip angles: accurate and precise measurements in the presence of radiofrequency field inhomogeneity. Magn Reson Med 55:566–574
Andreisek G, White LM, Yang Y, Robinson E, Cheng HL, Sussman MS (2009) Delayed gadolinium-enhanced MR imaging of articular cartilage: three-dimensional T1 mapping with variable flip angles and B1 correction. Radiology 252:865–873
Manuel A, Li W, Jellus V, Hughes T, Prasad PV (2011) Variable flip angle-based fast three-dimensional T1 mapping for delayed gadolinium-enhanced MRI of cartilage of the knee: need for B1 correction. Magn Reson Med 65:1377–1383
Sung K, Daniel BL, Hargreaves BA (2013) Transmit B1+ field inhomogeneity and T1 estimation errors in breast DCE-MRI at 3 tesla. J Magn Reson Imaging 38:454–459
Tofts PS, Brix G, Buckley DL et al (1999) Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging 10:223–232
Fritz-Hansen T, Rostrup E, Larsson HB, Søndergaard L, Ring P, Henriksen O (1996) Measurement of the arterial concentration of Gd-DTPA using MRI: a step toward quantitative perfusion imaging. Magn Reson Med 36:225–231
Othman AE, Martirosian P, Schraml C et al (2015) Feasibility of CAIPIRINHA-Dixon-TWIST-VIBE for dynamic contrast-enhanced MRI of the prostate. Eur J Radiol 84:2110–2116
Xie T, Zhao Q, Fu C et al (2018) Differentiation of triple-negative breast cancer from other subtypes through whole-tumor histogram analysis on multiparametric MR imaging. Eur Radiol. https://doi.org/10.1007/s00330-018-5804-5
Sinn HP, Kreipe H (2013) A brief overview of the WHO classification of breast tumors, 4th Edition, focusing on issues and updates from the 3rd edition. Breast Care (Basel) 8:149–154
Aerts HJ, Jaspers K, Backes WH (2011) The precision of pharmacokinetic parameters in dynamic contrast-enhanced magnetic resonance imaging: the effect of sampling frequency and duration. Phys Med Biol 56:5665–5678
Huang W, Tudorica LA, Li X et al (2011) Discrimination of benign and malignant breast lesions by using shutter-speed dynamic contrast-enhanced MR imaging. Radiology 261:394–403
Schabel MC, Morrell GR, Oh KY, Walczak CA, Barlow RB, Neumayer LA (2010) Pharmacokinetic mapping for lesion classification in dynamic breast MRI. J Magn Reson Imaging 31:1371–1378
Prat A, Cheang MC, Galvan P et al (2016) Prognostic value of intrinsic subtypes in hormone receptor-positive metastatic breast cancer treated with letrozole with or without lapatinib. JAMA Oncol 2:1287–1294
Harbeck N (2015) Insights into biology of luminal HER2 vs. enriched HER2 subtypes: therapeutic implications. Breast 24(Suppl 2):S44–S48
Shin JK, Kim JY (2017) Dynamic contrast-enhanced and diffusion-weighted MRI of estrogen receptor-positive invasive breast cancers: associations between quantitative MR parameters and Ki-67 proliferation status. J Magn Reson Imaging 45:94–102
Meng R, Chang SD, Jones EC, Goldenberg SL, Kozlowski P (2010) Comparison between population average and experimentally measured arterial input function in predicting biopsy results in prostate cancer. Acad Radiol 17:520–525
Li X, Welch EB, Arlinghaus LR et al (2011) A novel AIF tracking method and comparison of DCE-MRI parameters using individual and population-based AIFs in human breast cancer. Phys Med Biol 56:5753–5769
Funding
This study was funded by the National Natural Science Foundation of China (81801651).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Fuhua Yan.
Conflict of interest
The authors of this manuscript declare that they have relationships with Siemens Shenzhen Magnetic Resonance Ltd., Shenzhen, China, and Siemens Healthcare, Erlangen, Germany.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Institutional review board approval was obtained.
Ethical approval
Written informed consent was obtained from all subjects (patients) in this study.
Methodology
• Prospective
• Diagnostic or prognostic study
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 20 kb)
Rights and permissions
About this article
Cite this article
Sun, K., Zhu, H., Chai, W. et al. Whole-lesion histogram and texture analyses of breast lesions on inline quantitative DCE mapping with CAIPIRINHA-Dixon-TWIST-VIBE. Eur Radiol 30, 57–65 (2020). https://doi.org/10.1007/s00330-019-06365-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06365-8