Abstract
Objective
To evaluate the performance of quantitative computed tomography (CT) texture analysis using different machine learning (ML) classifiers for discriminating low and high nuclear grade clear cell renal cell carcinomas (cc-RCCs).
Materials and methods
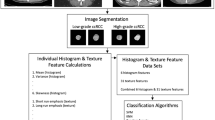
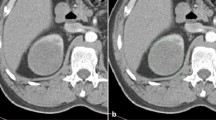
This retrospective study included 53 patients with pathologically proven 54 cc-RCCs (31 low-grade [grade 1 or 2]; 23 high-grade [grade 3 or 4]). In one patient, two synchronous cc-RCCs were included in the analysis. Mean age was 57.5 years. Thirty-four (64.1%) patients were male and 19 were female (35.9%). Mean tumour size based on the maximum diameter was 57.4 mm (range, 16–145 mm). Forty patients underwent radical nephrectomy and 13 underwent partial nephrectomy. Following pre-processing steps, two-dimensional CT texture features were extracted using portal-phase contrast-enhanced CT. Reproducibility of texture features was assessed with the intra-class correlation coefficient (ICC). Nested cross-validation with a wrapper-based algorithm was used in feature selection and model optimisation. The ML classifiers were support vector machine (SVM), multilayer perceptron (MLP, a sort of neural network), naïve Bayes, k-nearest neighbours, and random forest. The performance of the classifiers was compared by certain metrics.
Results
Among 279 texture features, 241 features with an ICC equal to or higher than 0.80 (excellent reproducibility) were included in the further feature selection process. The best model was created using SVM. The selected subset of features for SVM included five co-occurrence matrix (ICC range, 0.885–0.998), three run-length matrix (ICC range, 0.889–0.992), one gradient (ICC = 0.998), and four Haar wavelet features (ICC range, 0.941–0.997). The overall accuracy, sensitivity (for detecting high-grade cc-RCCs), specificity (for detecting high-grade cc-RCCs), and overall area under the curve of the best model were 85.1%, 91.3%, 80.6%, and 0.860, respectively.
Conclusions
The ML-based CT texture analysis can be a useful and promising non-invasive method for prediction of low and high Fuhrman nuclear grade cc-RCCs.
Key Points
• Based on the percutaneous biopsy literature, ML-based CT texture analysis has a comparable predictive performance with percutaneous biopsy.
• Highest predictive performance was obtained with use of the SVM.
• SVM correctly classified 85.1% of cc-RCCs in terms of nuclear grade, with an AUC of 0.860.





Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AUC:
-
Area under the curve
- cc-RCC:
-
Clear cell renal cell carcinoma
- CE-CT:
-
Contrast-enhanced computed tomography
- CT :
-
Computed tomography
- ICC:
-
Intra-class correlation coefficient
- ML:
-
Machine learning
- MLP:
-
Multilayer perceptron
- RCC:
-
Renal cell carcinoma
- ROC:
-
Receiver operating characteristic
- ROI:
-
Region of interest
- SVM:
-
Support vector machine
References
Eble J, Sauter G, Epstein J, Sesterhenn I (2004) Pathology and genetics of tumours of the urinary system and male genital organs. WHO Classification of Tumours. IARC Press, Lyon, France, pp 9–11
Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, Bray F (2015) International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol 67:519–530. https://doi.org/10.1016/j.eururo.2014.10.002
Gupta K, Miller JD, Li JZ, Russell MW, Charbonneau C (2008) Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev 34:193–205. https://doi.org/10.1016/j.ctrv.2007.12.001
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ (2009) Cancer statistics, 2009. CA Cancer J Clin 59:225–249. https://doi.org/10.3322/caac.20006
Lohse CM, Blute ML, Zincke H, Weaver AL, Cheville JC (2002) Comparison of standardized and nonstandardized nuclear grade of renal cell carcinoma to predict outcome among 2,042 patients. Am J Clin Pathol 118:877–886. https://doi.org/10.1309/VLV6-BRTR-HY5B-H485
Minardi D, Lucarini G, Mazzucchelli R et al (2005) Prognostic role of Fuhrman grade and vascular endothelial growth factor in pT1a clear cell carcinoma in partial nephrectomy specimens. J Urol 174:1208–1212
Fuhrman SA, Lasky LC, Limas C (1982) Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol 6:655–663
Delahunt B (2009) Advances and controversies in grading and staging of renal cell carcinoma. Mod Pathol 22:S24–S36. https://doi.org/10.1038/modpathol.2008.183
Campbell N, Rosenkrantz AB, Pedrosa I (2014) MRI phenotype in renal cancer. Top Magn Reson Imaging 23:85–105. https://doi.org/10.1097/RMR.0000000000000019
Kunkle DA, Egleston BL, Uzzo RG (2008) Excise, ablate or observe: the small renal mass dilemma--a meta-analysis and review. J Urol 179:1227–1233; discussion 1233–4. https://doi.org/10.1016/j.juro.2007.11.047
Jewett MA, Mattar K, Basiuk J et al (2011) Active surveillance of small renal masses: progression patterns of early stage kidney cancer. Eur Urol 60:39–44. https://doi.org/10.1016/j.eururo.2011.03.030
Marconi L, Dabestani S, Lam TB et al (2016) Systematic review and meta-analysis of diagnostic accuracy of percutaneous renal tumour biopsy. Eur Urol 69:660–673. https://doi.org/10.1016/j.eururo.2015.07.072
Volpe A, Mattar K, Finelli A et al (2008) Contemporary results of percutaneous biopsy of 100 small renal masses: a single centre experience. J Urol 180:2333–2337. https://doi.org/10.1016/j.juro.2008.08.014
Parada Villavicencio C, Mc Carthy RJ, Miller FH (2017) Can diffusion-weighted magnetic resonance imaging of clear cell renal carcinoma predict low from high nuclear grade tumours. Abdom Radiol (NY) 42:1241–1249. https://doi.org/10.1007/s00261-016-0981-7
Rosenkrantz AB, Niver BE, Fitzgerald EF, Babb JS, Chandarana H, Melamed J (2010) Utility of the apparent diffusion coefficient for distinguishing clear cell renal cell carcinoma of low and high nuclear grade. AJR Am J Roentgenol 195:W344–W351. https://doi.org/10.2214/AJR.10.4688
Schieda N, Lim RS, Krishna S, McInnes MDF, Flood TA, Thornhill RE (2018) Diagnostic accuracy of unenhanced CT analysis to differentiate low-grade from high-grade chromophobe renal cell carcinoma. AJR Am J Roentgenol 1–9. doi: https://doi.org/10.2214/AJR.17.18874
Ding J, Xing Z, Jiang Z et al (2018) CT-based radiomic model predicts high grade of clear cell renal cell carcinoma. Eur J Radiol 103:51–56. https://doi.org/10.1016/J.EJRAD.2018.04.013
Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: images are more than pictures, they are data. Radiology 278:563–577. https://doi.org/10.1148/radiol.2015151169
Aerts HJWL, Velazquez ER, Leijenaar RTH et al (2014) Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 5:4006. https://doi.org/10.1038/ncomms5006
Davnall F, Yip CSP, Ljungqvist G et al (2012) Assessment of tumour heterogeneity: an emerging imaging tool for clinical practice? Insights Imaging 3:573–589. https://doi.org/10.1007/s13244-012-0196-6
Feng Z, Rong P, Cao P et al (2018) Machine learning-based quantitative texture analysis of CT images of small renal masses: differentiation of angiomyolipoma without visible fat from renal cell carcinoma. Eur Radiol 28:1625–1633. https://doi.org/10.1007/s00330-017-5118-z
Kumar V, Gu Y, Basu S et al (2012) Radiomics: the process and the challenges. Magn Reson Imaging 30:1234–1248. https://doi.org/10.1016/j.mri.2012.06.010
Parmar C, Grossmann P, Bussink J et al (2015) Machine learning methods for quantitative radiomic biomarkers. Sci Rep 5:13087. https://doi.org/10.1038/srep13087
Zhang X, Yan L-F, Hu Y-C et al (2017) Optimizing a machine learning based glioma grading system using multi-parametric MRI histogram and texture features. Oncotarget 8:47816–47830. https://doi.org/10.18632/oncotarget.18001
Mannil M, Burgstaller JM, Thanabalasingam A et al (2018) Texture analysis of paraspinal musculature in MRI of the lumbar spine: analysis of the lumbar stenosis outcome study (LSOS) data. Skeletal Radiol. https://doi.org/10.1007/s00256-018-2919-3
Shafiq-ul-Hassan M, Zhang GG, Latifi K et al (2017) Intrinsic dependencies of CT radiomic features on voxel size and number of gray levels. Med Phys 44:1050–1062. https://doi.org/10.1002/mp.12123
Collewet G, Strzelecki M, Mariette F (2004) Influence of MRI acquisition protocols and image intensity normalization methods on texture classification. Magn Reson Imaging 22:81–91. https://doi.org/10.1016/j.mri.2003.09.001
Szczypinski PM, Strzelecki M, Materka A (2007) Mazda - a software for texture analysis. In: 2007 International Symposium on Information Technology Convergence (ISITC 2007). IEEE, pp 245–249
Szczypiński PM, Strzelecki M, Materka A, Klepaczko A (2009) MaZda—A software package for image texture analysis. Comput Methods Programs Biomed 94:66–76. https://doi.org/10.1016/j.cmpb.2008.08.005
Strzelecki M, Szczypinski P, Materka A, Klepaczko A (2013) A software tool for automatic classification and segmentation of 2D/3D medical images. Nucl Instruments Methods Phys Res Sect A Accel Spectrometers, Detect Assoc Equip 702:137–140. https://doi.org/10.1016/j.nima.2012.09.006
Nketiah G, Elschot M, Kim E et al (2017) T2-weighted MRI-derived textural features reflect prostate cancer aggressiveness: preliminary results. Eur Radiol 27:3050–3059. https://doi.org/10.1007/s00330-016-4663-1
Wibmer A, Hricak H, Gondo T et al (2015) Haralick texture analysis of prostate MRI: utility for differentiating non-cancerous prostate from prostate cancer and differentiating prostate cancers with different Gleason scores. Eur Radiol 25:2840–2850. https://doi.org/10.1007/s00330-015-3701-8
Xu J-W, Suzuki K (2014) Max-AUC feature selection in computer-aided detection of polyps in CT colonography. IEEE J Biomed Heal informatics 18:585–593. https://doi.org/10.1109/JBHI.2013.2278023
Mwangi B, Tian TS, Soares JC (2014) A review of feature reduction techniques in neuroimaging. Neuroinformatics 12:229–244. https://doi.org/10.1007/s12021-013-9204-3
Cawley GC, Talbot NLC (2010) On over-fitting in model selection and subsequent selection bias in performance evaluation. J Mach Learn Res 11:2079–2107
Kohavi R, John GH (1997) Wrappers for feature subset selection. Artif Intell 97:273–324. https://doi.org/10.1016/S0004-3702(97)00043-X
Bermejo P, Gamez JA, Puerta JM (2011) Improving incremental wrapper-based subset selection via replacement and early stopping. Int J Pattern Recognit Artif Intell 25:605–625. https://doi.org/10.1142/S0218001411008804
Ahmad A, Dey L (2005) A feature selection technique for classificatory analysis. Pattern Recognit Lett 26:43–56. https://doi.org/10.1016/j.patrec.2004.08.015
Erickson BJ, Korfiatis P, Akkus Z, Kline TL (2017) Machine learning for medical imaging. Radiographics 37:505–515. https://doi.org/10.1148/rg.2017160130
Kierans AS, Rusinek H, Lee A et al (2014) Textural differences in apparent diffusion coefficient between low- and high-stage clear cell renal cell carcinoma. AJR Am J Roentgenol 203:W637–W644. https://doi.org/10.2214/AJR.14.12570
Woo S, Suh CH, Kim SY et al (2017) Diagnostic performance of DWI for differentiating high- from low-grade clear cell renal cell carcinoma: a systematic review and meta-analysis. AJR Am J Roentgenol 209:W374–W381. https://doi.org/10.2214/AJR.17.18283
Ng F, Kozarski R, Ganeshan B, Goh V (2013) Assessment of tumour heterogeneity by CT texture analysis: can the largest cross-sectional area be used as an alternative to whole tumour analysis? Eur J Radiol 82:342–348. https://doi.org/10.1016/j.ejrad.2012.10.023
Klatte T, Patard J-J, de Martino M et al (2008) tumour size does not predict risk of metastatic disease or prognosis of small renal cell carcinomas. J Urol 179:1719–1726. https://doi.org/10.1016/j.juro.2008.01.018
Frank I, Blute ML, Cheville JC et al (2002) An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumour stage, size, grade and necrosis: the SSIGN score. J Urol 168:2395–2400. https://doi.org/10.1097/01.ju.0000035885.91935.d5
Zisman A, Pantuck AJ, Dorey F et al (2002) Mathematical model to predict individual survival for patients with renal cell carcinoma. J Clin Oncol 20:1368–1374. https://doi.org/10.1200/JCO.2002.20.5.1368
Abou Youssif T, Tanguay S (2009) Natural history and management of small renal masses. Curr Oncol 16(Suppl 1):S2–S7
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Burak Kocak, MD.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
Burak Kocak, MD, the second and corresponding author, has significant statistical expertise.
Informed consent
Written informed consent was waived by the institutional review board.
Ethical approval
Institutional review board approval was obtained.
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Rights and permissions
About this article
Cite this article
Bektas, C.T., Kocak, B., Yardimci, A.H. et al. Clear Cell Renal Cell Carcinoma: Machine Learning-Based Quantitative Computed Tomography Texture Analysis for Prediction of Fuhrman Nuclear Grade. Eur Radiol 29, 1153–1163 (2019). https://doi.org/10.1007/s00330-018-5698-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5698-2