Abstract
Objectives
To develop and validate a clinical-radiomics nomogram for preoperative prediction of lung metastasis for colorectal cancer (CRC) patients with indeterminate pulmonary nodules (IPN).
Methods
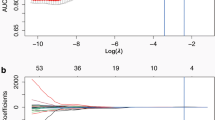
194 CRC patients with lung nodules were enrolled in this study (136 in the training cohort and 58 in the validation cohort). To evaluate the probability of lung metastasis, we developed three models, the clinical model with significant clinical risk factors, the radiomics model with radiomics features constructed by the least absolute shrinkage and selection operator algorithm, and the clinical-radiomics model with significant variables selected by the stepwise logistic regression. The Akaike information criterion (AIC) was used to compare the relative strength of different models, and the area under the curve (AUC) was used to quantify the predictive accuracy. The nomogram was developed based on the most appropriate model. Decision-curve analysis was applied to assess the clinical usefulness.
Results
The clinical-radiomics model (AIC = 98.893) with the lowest AIC value compared with that of the clinical-only model (AIC = 138.502) or the radiomics-only model (AIC = 116.146) was identified as the best model. The clinical-radiomics nomogram was also successfully developed with favourable discrimination in both training cohort (AUC = 0.929, 95% CI: 0.885–0.974) and validation cohort (AUC = 0.922, 95% CI: 0.857–0.986), and good calibration. Decision-curve analysis confirmed the clinical utility of the clinical-radiomics nomogram.
Conclusions
In CRC patients with IPNs, the clinical-radiomics nomogram created by the radiomics signature and clinical risk factors exhibited favourable discriminatory ability and accuracy for a metastasis prediction.
Key Points
• Clinical features can predict lung metastasis of colorectal cancer patients.
• Radiomics analysis outperformed clinical features in assessing the risk of pulmonary metastasis.
• A clinical-radiomics nomogram can help clinicians predict lung metastasis in colorectal cancer patients.





Similar content being viewed by others
Abbreviations
- AIC:
-
Akaike information criterion
- AUC:
-
Area under the curve
- CA19-9:
-
Carbohydrate antigen 19-9
- CEA:
-
Carcinoembryonic antigen
- CI:
-
Confidence interval
- CRC:
-
Colorectal cancer
- DCA:
-
Decision curve analysis
- IPN:
-
Indeterminate pulmonary nodules
- ITT:
-
Intravascular tumour thrombus
- LASSO:
-
Least absolute shrinkage and selection operator
- LM:
-
Lung metastasis
- LR test:
-
Likelihood-ratio test
- NM:
-
Non-metastasis
- NPV:
-
Negative predictive value
- PNI:
-
Perineural invasion
- PPV:
-
Positive predictive value
- ROC:
-
Receiver operating characteristic curve
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65:87–108
Kobayashi H, Mochizuki H, Sugihara K et al (2007) Characteristics of recurrence and surveillance tools after curative resection for colorectal cancer: a multicenter study. Surgery 141:67–75
Desch CE, Benson AB 3rd, Somerfield MR et al (2005) Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol 23:8512–8519
Vachani A, Tanner NT, Aggarwal J et al (2014) Factors that influence physician decision making for indeterminate pulmonary nodules. Ann Am Thorac Soc 11:1586–1591
NM A (2003) The solitary pulmonary nodule. N Engl J Med 349(16):1575
Inoue M, Ohta M, Iuchi K et al (2004) Benefits of surgery for patients with pulmonary metastases from colorectal carcinoma. Ann Thorac Surg 78:238–244
De Wever W, Meylaerts L, De Ceuninck L, Stroobants S, Verschakelen JA (2007) Additional value of integrated PET-CT in the detection and characterization of lung metastases: correlation with CT alone and PET alone. Eur Radiol 17:467–473
Aerts HJ, Velazquez ER, Leijenaar RT et al (2014) Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 5:4006
Zhang Y, Moore GR, Laule C et al (2013) Pathological correlates of magnetic resonance imaging texture heterogeneity in multiple sclerosis. Ann Neurol 74:91–99
Yoon HJ, Kim Y, Kim BS (2015) Intratumoral metabolic heterogeneity predicts invasive components in breast ductal carcinoma in situ. Eur Radiol 25:3648–3658
Win T, Miles KA, Janes SM et al (2013) Tumor heterogeneity and permeability as measured on the CT component of PET/CT predict survival in patients with non-small cell lung cancer. Clin Cancer Res 19:3591–3599
Ganeshan B, Goh V, Mandeville HC (2013) Non-small cell lung cancer: histopathologic correlates for texture parameters at CT. Radiology 266:326–336
Ahmed A, Gibbs P, Pickles M, Turnbull L (2013) Texture analysis in assessment and prediction of chemotherapy response in breast cancer. J Magn Reson Imaging 38:89–101
Baek SJ, Kim SH, Kwak JM et al (2012) Indeterminate pulmonary nodules in rectal cancer: a recommendation for follow-up guidelines. J Surg Oncol 106:481–485
Kim CH, Huh JW, Kim HR, Kim YJ (2015) Indeterminate pulmonary nodules in colorectal cancer: follow-up guidelines based on a risk predictive model. Ann Surg 261:1145–1152
Mitry E, Guiu B, Cosconea S, Jooste V, Faivre J, Bouvier AM (2010) Epidemiology, management and prognosis of colorectal cancer with lung metastases: a 30-year population-based study. Gut 59:1383–1388
Cufer T, Ovcaricek T, O’Brien ME (2013) Systemic therapy of advanced non-small cell lung cancer: major-developments of the last 5-years. Eur J Cancer 49:1216–1225
Kumar V, Gu Y, Basu S et al (2012) Radiomics: the process and the challenges. Magn Reson Imaging 30:1234–1248
Wei K, Su H, Zhou G et al (2016) Potential Application of Radiomics for Differentiating Solitary Pulmonary Nodules. OMICS J Radiol 5:1-5
Park J, Kobayashi Y, Urayama KY, Yamaura H, Yatabe Y, Hida T (2016) Imaging Characteristics of Driver Mutations in EGFR, KRAS, and ALK among Treatment-Naive Patients with Advanced Lung Adenocarcinoma. PLoS One 11:e0161081
Divine MR, Katiyar P, Kohlhofer U, Quintanilla-Martinez L, Pichler BJ, Disselhorst JA (2016) A Population-Based Gaussian Mixture Model Incorporating 18F-FDG PET and Diffusion-Weighted MRI Quantifies Tumor Tissue Classes. J Nucl Med 57:473–479
Wu W, Parmar C, Grossmann P et al (2016) Exploratory study to identify radiomics classifiers for lung cancer histology. Front Oncol 6:71
Kamiya A, Murayama S, Kamiya H, Yamashiro T, Oshiro Y, Tanaka N (2014) Kurtosis and skewness assessments of solid lung nodule density histograms: differentiating malignant from benign nodules on CT. Jpn J Radiol 32:14–21
Ko JP, Suh J, Ibidapo O et al (2016) Lung adenocarcinoma: correlation of quantitative CT findings with pathologic findings. Radiology 280:931–939
Son JY, Lee HY, Kim JH et al (2016) Quantitative CT analysis of pulmonary ground-glass opacity nodules for distinguishing invasive adenocarcinoma from non-invasive or minimally invasive adenocarcinoma: the added value of using iodine mapping. Eur Radiol 26:43–54
Hanania AN, Bantis LE, Feng Z et al (2016) Quantitative imaging to evaluate malignant potential of IPMNs. Oncotarget 7:85776–85784
Lee G, Lee HY, Park H et al (2017) Radiomics and its emerging role in lung cancer research, imaging biomarkers and clinical management: State of the art. Eur J Radiol 86:297–307
Funding
This study has received funding by the National Science Foundation for Young Scientists of China (Grant No.81501437).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Tong Tong.
Conflict of interest
The authors of this article declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
Shengping Wang has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• observational
• performed at one institution
Electronic supplementary material
ESM 1
(DOCX 5286 kb)
Rights and permissions
About this article
Cite this article
Hu, T., Wang, S., Huang, L. et al. A clinical-radiomics nomogram for the preoperative prediction of lung metastasis in colorectal cancer patients with indeterminate pulmonary nodules. Eur Radiol 29, 439–449 (2019). https://doi.org/10.1007/s00330-018-5539-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5539-3