Abstract
Objectives
The aim of this pilot study was to investigate the utility of haemodynamic parameters derived from dynamic contrast-enhanced computed tomography (DCE-CT) scans in the assessment of tumour response to treatment in malignant pleural mesothelioma (MPM) patients.
Methods
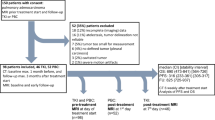
The patient cohort included nine patients undergoing chemotherapy and five patients on observation. Each patient underwent two DCE-CT scans separated by approximately 2 months. The DCE-CT parameters of tissue blood flow (BF) and tissue blood volume (BV) were obtained within the dynamically imaged tumour. Mean relative changes in tumour DCE-CT parameters between scans were compared between the on-treatment and on-observation cohorts. DCE-CT parameter changes were correlated with relative change in tumour bulk evaluated according to the modified RECIST protocol.
Results
Differing trends in relative change in BF and BV between scans were found between the two patient groups (p = 0.19 and p = 0.06 for BF and BV, respectively). No significant rank correlations were found when comparing relative changes in DCE-CT parameters with relative change in tumour bulk.
Conclusions
Differing trends in the relative change of BF and BV between patients on treatment and on observation indicate the potential of DCE-CT for the assessment of pharmacodynamic endpoints with respect to treatment in MPM. A future study with a larger patient cohort and unified treatment regimens should be undertaken to confirm the results of this pilot study.
Key Points
• CT-derived haemodynamic parameters show differing trends between malignant pleural mesothelioma patients on treatment and patients off treatment
• Changes in haemodynamic parameters do not correlate with changes in tumour bulk as measured according to the modified RECIST protocol
• Differing trends across the two patient groups indicate the potential sensitivity of DCE-CT to assess pharmacodynamic endpoints in the treatment of MPM



Similar content being viewed by others
Abbreviations
- BF:
-
Tissue blood flow
- BV:
-
Tissue blood volume
- CI:
-
Confidence interval
- DCE-CT:
-
Dynamic contrast-enhanced computed tomography
- MPM:
-
Malignant pleural mesothelioma
- RECIST:
-
Response Evaluation Criteria in Solid Tumours
References
van Meerbeeck JP, Scherpereel A, Surmont VF, Baas P (2011) Malignant pleural mesothelioma: the standard of care and challenges for future management. Crit Rev Oncol Hematol 78:92–111
Vogelzang BNJ, Rusthoven JJ, Symanowski J et al (2008) Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural. J Clin Oncol 21:2636–2644
Nickell LT, Lichtenberger Iii JP, Khorashadi L et al (2014) Multimodality imaging for characterization, classification, and staging of malignant pleural mesothelioma. Radiographics 34:1692–1706
Byrne MJ, Nowak AK (2004) Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol 15:257–260
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216
Oxnard GR, Armato SG III, Kindler HL (2006) Modeling of mesothelioma growth demonstrates weaknesses of current response criteria. Lung Cancer 52:141–148
Liu F, Zhao B, Krug LM et al (2010) Assessment of therapy responses and prediction of survival in malignant pleural mesothelioma through computer-aided volumetric measurement on computed tomography scans. J Thorac Oncol 5:879–884
Frauenfelder T, Tutic M, Weder W et al (2011) Volumetry: an alternative to assess therapy response for malignant pleural mesothelioma? Eur Respir J 38:162–168
Labby ZE, Nowak AK, Dignam JJ et al (2013) Disease volumes as a marker for patient response in malignant pleural mesothelioma. Ann Oncol 24:999–1005
Remon J, Reguart N, Corral J, Lianes P (2015) Malignant pleural mesothelioma: new hope in the horizon with novel therapeutic strategies. Cancer Treat Rev 41:27–34
Kindler HL, Karrison TG, Gandara DR et al (2012) Multicenter, double-blind, placebo-controlled, randomized phase II trial of gemcitabine/cisplatin plus bevacizumab or placebo in patients with malignant mesothelioma. J Clin Oncol 30:2509–2515
Zalcman G, Mazieres J, Margery J et al (2016) Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet 387:1405–1414
Wolchok JD, Hoos A, O’Day S et al (2009) Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 15:7412–7420
Thiam R, Fournier LS, Trinquart L et al (2010) Optimizing the size variation threshold for the CT evaluation of response in metastatic renal cell carcinoma treated with sunitinib. Ann Oncol 21:936–941
Nishino M, Jagannathan JP, Krajewski KM et al (2012) Personalized tumor response assessment in the era of molecular medicine: cancer-specific and therapy-specific response criteria to complement pitfalls of RECIST. AJR Am J Roentgenol 198:737
Prezzi D, Khan A, Goh V (2015) Perfusion CT imaging of treatment response in oncology. Eur J Radiol 84:2380–2385
Blomley MJ, Coulden R, Bufkin C et al (1993) Contrast bolus dynamic computed tomography for the measurement of solid organ perfusion. Invest Radiol 28:72–77
Miles KA, Griffiths MR (2003) Perfusion CT: a worthwhile enhancement? Br J Radiol 76:220–231
Sudarski S, Shi J, Schmid-Bindert G et al (2015) Dynamic volume perfusion computed tomography parameters versus RECIST for the prediction of outcome in lung cancer patients treated with conventional chemotherapy. J Thorac Oncol 10:164–171
Yang L, Zhang X, Tan B et al (2012) Computed tomographic perfusion imaging for the therapeutic response of chemoembolization for hepatocellular carcinoma. J Comput Assist Tomogr 36:226–230
Christner JA, Kofler JM, McCollough CH (2010) Estimating effective dose for CT using dose–length product compared with using organ doses: consequences of adopting International Commission on Radiological Protection Publication 103 or dual-energy scanning. Am J Roentgenol 194:881–889
Huda W, Ogden KM, Khorasani MR (2008) Converting dose-length product to effective dose at CT. Radiology 248:995–1003
Avants BB, Tustison NJ, Song G et al (2011) A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 54:2033–2044
Axel L (1980) Cerebral blood flow determination by rapid-sequence computed tomography: theoretical analysis. Radiology 137:679–686
Koenig M, Klotz E, Luka B et al (1998) Perfusion CT of the brain: diagnostic approach for early detection of ischemic stroke. Radiology 209:85–93
Hodges JL Jr, Lehmann EL (1963) Estimates of location based on rank tests. Ann Math Stat 598–611
Bauer DF (1972) Constructing confidence sets using rank statistics. J Am Stat Assoc 67:687–690
Abdi H (2007) The Bonferonni and Šidák corrections for multiple comparisons. Enc Meas Stat 3:103–107
Meijerink MR, van Cruijsen H, Hoekman K et al (2007) The use of perfusion CT for the evaluation of therapy combining AZD2171 with gefitinib in cancer patients. Eur Radiol 17:1700–1713
Acknowledgements
The authors would like to thank The University of Chicago’s Human Imaging Research Office (HIRO) for their assistance in coordinating the imaging aspects of this study and providing anonymised, compliant images for evaluation. The HIRO is supported in part by pilot research funding provided by the Virginia and D. K. Ludwig Fund for Cancer Research via the Imaging Research Institute in the Biological Sciences Division of the University of Chicago and through Cancer Center Support Grant number P30 CA014599 from the University of Chicago Comprehensive Cancer Center.
Funding
This study has received funding by the Hodges Society of the Department of Radiology at The University of Chicago and the Plooy Family and the Kazan McClain Partners’ Foundation, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Samuel G. Armato III, Ph.D., at the University of Chicago.
Conflict of interest
The authors of this manuscript declare relationships with the following companies: SGA receives royalties and licensing fees for computer-aided diagnostic technology through the University of Chicago. SGA is a consultant for Aduro Biotech, Inc.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional review board approval was obtained.
Methodology
• prospective
• diagnostic or prognostic study
• performed at one institution
Rights and permissions
About this article
Cite this article
Gudmundsson, E., Labby, Z., Straus, C.M. et al. Dynamic contrast-enhanced CT for the assessment of tumour response in malignant pleural mesothelioma: a pilot study. Eur Radiol 29, 682–688 (2019). https://doi.org/10.1007/s00330-018-5533-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5533-9