Abstract
Objectives
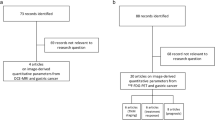
To investigate usefulness of multiparametric fully integrated 18-FDG PET/MRI in predicting treatment response after chemotherapy for unresectable advanced gastric cancers (AGCs).
Methods
Eleven patients with unresectable AGCs underwent multiparametric 18-FDG PET/MRI examinations prior to chemotherapy. Perfusion parameters obtained via dynamic contrast-enhanced MRI, apparent diffusion coefficient values from diffusion-weighted images, and maximum standardized uptake values (SUVmax) from 18-FDG PET were measured. For parameters obtained from 18-FDG PET/MRI data, interobserver agreement was obtained using intraclass correlation coefficients (ICC) and chemotherapy response relationship was evaluated using the Mann–Whitney test and receiver operating characteristic analysis.
Results
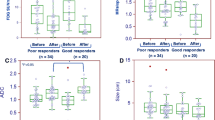
After chemotherapy, six patients were classified into the responder group and five patients into the non-responder group. For all parameters, moderate to nearly perfect agreement was achieved (ICC = 0.452–0.911). K trans values (P = 0.018) and initial area under the curves (iAUCs) (P = 0.045) of gastric cancers were significantly higher in responder group than in non-responder group. The area under the curve was 0.917 for K trans and 0.867 for iAUC. However, SUVmax values were not significantly different between the two groups.
Conclusion
Multiparametric approach using fully integrated 18-FDG PET/MRI was shown to be feasible for patients with unresectable gastric cancers. In addition, K trans and iAUC values can be used as early predictive markers for chemotherapy response.
Key Points:
• Multiparametric 18-FDG PET/MRI is feasible for patients with unresectable advanced gastric cancer
• K trans and iAUC were significantly higher in the responder group of patients
• K trans , iAUC can be utilized as early predictive markers for chemotherapeutic response



Similar content being viewed by others
References
Greenlee RT, Murray T, Bolden S, Wingo PA (2000) Cancer statistics, 2000. CA Cancer J Clin 50:7–33
Soerjomataram I, Lortet-Tieulent J, Parkin DM et al (2012) Global burden of cancer in 2008: a systematic analysis of disability-adjusted life-years in 12 world regions. Lancet 380:1840–1850
Wohrer SS, Raderer M, Hejna M (2004) Palliative chemotherapy for advanced gastric cancer. Ann Oncol 15:1585–1595
Glimelius B, Ekstrom K, Hoffman K et al (1997) Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol 8:163–168
Cascorbi I (2010) The promises of personalized medicine. Eur J Clin Pharmacol 66:749–754
Collins CD, Purohit S, Podolsky RH et al (2006) The application of genomic and proteomic technologies in predictive, preventive and personalized medicine. Vasc Pharmacol 45:258–267
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Chung HH, Kim JW, Han KH et al (2011) Prognostic value of metabolic tumor volume measured by FDG-PET/CT in patients with cervical cancer. Gynecol Oncol 120:270–274
Lee HY, Hyun SH, Lee KS et al (2010) Volume-based parameter of 18)F-FDG PET/CT in malignant pleural mesothelioma: prediction of therapeutic response and prognostic implications. Ann Surg Oncol 17:2787–2794
Harry VN, Semple SI, Parkin DE, Gilbert FJ (2010) Use of new imaging techniques to predict tumour response to therapy. Lancet Oncol 11:92–102
Koh TS, Thng CH, Lee PS et al (2008) Hepatic metastases: in vivo assessment of perfusion parameters at dynamic contrast-enhanced MR imaging with dual-input two-compartment tracer kinetics model. Radiology 249:307–320
Rischpler C, Nekolla SG, Kunze KP, Schwaiger M (2015) PET/MRI of the heart. Semin Nucl Med 45:234–247
Bagade S, Fowler KJ, Schwarz JK, Grigsby PW, Dehdashti F (2015) PET/MRI evaluation of gynecologic malignancies and prostate cancer. Semin Nucl Med 45:293–303
Buchbender C, Heusner TA, Lauenstein TC, Bockisch A, Antoch G (2012) Oncologic PET/MRI, part 2: bone tumors, soft-tissue tumors, melanoma, and lymphoma. J Nucl Med 53:1244–1252
Buchbender C, Heusner TA, Lauenstein TC, Bockisch A, Antoch G (2012) Oncologic PET/MRI, part 1: tumors of the brain, head and neck, chest, abdomen, and pelvis. J Nucl Med 53:928–938
Joo I, Lee JM, Han JK, Yang HK, Lee HJ, Choi BI (2014) Dynamic contrast-enhanced MRI of gastric cancer: correlation of the perfusion parameters with pathological prognostic factors. J Magn Reson Imaging 41:1608–1614
Kim JH, Lee JM, Park JH et al (2013) Solid pancreatic lesions: characterization by using timing bolus dynamic contrast-enhanced MR imaging assessment–a preliminary study. Radiology 266:185–196
Tofts PS, Kermode AG (1991) Measurement of the blood-brain barrier permeability and leakage space using dynamic MR imaging. 1. Fundamental concepts. Magn Reson Med 17:357–367
Tofts PS, Brix G, Buckley DL et al (1999) Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging 10:223–232
Orton MR, d'Arcy JA, Walker-Samuel S et al (2008) Computationally efficient vascular input function models for quantitative kinetic modelling using DCE–MRI. Phys Med Biol 53:1225–1239
Partovi S, Kohan A, Rubbert C et al (2014) Clinical oncologic applications of PET/MRI: a new horizon. Am J Nucl Med Mol Imaging 4:202–212
Song HK, Dougherty L (2000) k-space weighted image contrast (KWIC) for contrast manipulation in projection reconstruction MRI. Magn Reson Med 44:825–832
Kim KW, Lee JM, Jeon YS et al (2013) Free-breathing dynamic contrast-enhanced MRI of the abdomen and chest using a radial gradient echo sequence with K-space weighted image contrast (KWIC). Eur Radiol 23:1352–1360
Turkbey B, Thomasson D, Pang Y, Bernardo M, Choyke PL (2010) The role of dynamic contrast-enhanced MRI in cancer diagnosis and treatment. Diagn Interv Radiol 16:186–192
Cooper RA, Carrington BM, Loncaster JA et al (2000) Tumour oxygenation levels correlate with dynamic contrast-enhanced magnetic resonance imaging parameters in carcinoma of the cervix. Radiother Oncol 57:53–59
Lim JS, Kim D, Baek SE et al (2012) Perfusion MRI for the prediction of treatment response after preoperative chemoradiotherapy in locally advanced rectal cancer. Eur Radiol 22:1693–1700
Kim SH, Lee JM, Gupta SN, Han JK, Choi BI (2014) Dynamic contrast-enhanced MRI to evaluate the therapeutic response to neoadjuvant chemoradiation therapy in locally advanced rectal cancer. J Magn Reson Imaging 40:730–737
Roberts C, Issa B, Stone A, Jackson A, Waterton JC, Parker GJ (2006) Comparative study into the robustness of compartmental modeling and model-free analysis in DCE–MRI studies. J Magn Reson Imaging 23:554–563
Milosevic M, Fyles A, Hedley D et al (2001) Interstitial fluid pressure predicts survival in patients with cervix cancer independent of clinical prognostic factors and tumor oxygen measurements. Cancer Res 61:6400–6405
Chung HW, Lee EJ, Cho YH et al (2010) High FDG uptake in PET/CT predicts worse prognosis in patients with metastatic gastric adenocarcinoma. J Cancer Res Clin Oncol 136:1929–1935
Ott K, Herrmann K, Lordick F et al (2008) Early metabolic response evaluation by fluorine-18 fluorodeoxyglucose positron emission tomography allows in vivo testing of chemosensitivity in gastric cancer: long-term results of a prospective study. Clin Cancer Res 14:2012–2018
Di Fabio F, Pinto C, Rojas Llimpe FL et al (2007) The predictive value of 18F-FDG-PET early evaluation in patients with metastatic gastric adenocarcinoma treated with chemotherapy plus cetuximab. Gastric Cancer 10:221–227
Ott K, Fink U, Becker K et al (2003) Prediction of response to preoperative chemotherapy in gastric carcinoma by metabolic imaging: results of a prospective trial. J Clin Oncol 21:4604–4610
Acknowledgements
The scientific guarantor of this publication is Se Hyung Kim. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this study was supported by a grant from the Seoul National University Hospital Research Fund No. 03-2013-390. No complex statistical methods were necessary for this paper. Institutional review board approval was obtained. Written informed consent was obtained from all patients in this study. Methodology: prospective, observational, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Lee, D.H., Kim, S.H., Im, SA. et al. Multiparametric fully-integrated 18-FDG PET/MRI of advanced gastric cancer for prediction of chemotherapy response: a preliminary study. Eur Radiol 26, 2771–2778 (2016). https://doi.org/10.1007/s00330-015-4105-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-4105-5