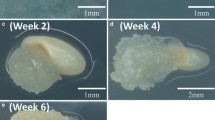
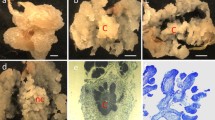
Abstract
The increasing interest in renewable energy has attracted more research attention on biofuels. In order to generate sustainable amount of biomass feedstock from dedicated biofuel crops such as switchgrass they need to be genetically improved. Genetic transformation is one of the techniques to achieve this goal. The aim of our study was to devise a simplified protocol for switchgrass genetic transformation. We have used NB0 as the basal medium and mature seeds of the cultivar Alamo as the starting material. The nutrient medium used and scutellum-derived callus are fashioned after rice genetic transformation protocols. We obtained friable calluses, which were similar to the type II calluses in other monocotyledonous species. Calluses were amenable for Agrobacterium-mediated genetic transformation with at least 6 % transformation efficiency. The concentration of hygromycin was optimized for successful selection of transgenic calluses. The Green Fluorescent Protein gene was used to monitor and demonstrate successful genetic transformation. Compared to the previously published methods for genetic transformation of switchgrass, our protocol is simpler and equally efficient.
Key message
An efficient, simplified switchgrass genetic transformation method with NB0 basal medium and mature seeds as inoculum was developed. The appropriate concentrations of hormones and selection agent are described.






Similar content being viewed by others
Abbreviations
- 2,4-D :
-
2,4-Dichlorophenoxyacetic acid
- BA:
-
N 6-Benzyladenine
- sGFP:
-
Synthetic green fluorescent protein
- HPT:
-
Hygromycin phosphotransferase
- KOH:
-
Potassium hydroxide
- MS:
-
Murashige and Skoog
References
Abalaka ME, Mohammed A (2011) Agrobacterium transformation: a boost to agricultural biotechnology. J Med Genet Genomics 3:126–130
Birch RG (1997) Plant transformation: problems and strategies for practical application. Annu Rev Plant Physiol Plant Mol Biol 48:297–326
Burris JN, Mann DGJ, Joyce BL, Stewart CN (2009) An Improved tissue culture system for embryogenic callus production and plant regeneration in switchgrass (Panicum virgatum L.). Bioenerg Res 2:267–274
Chiu W, Niwa Y, Zeng W, Hirano T, Kobayashi H, Sheen J (1996) Engineered GFP as a vital reporter in plants. Curr Biol 6:325–330
Chu CC, Wang CC, Sun CS (1978) The N6 medium and its application to anther culture of cereal crops. In: Proceedings of symposium on plant tissue culture. Science Press, Peking, pp 45–50
Fu C, Mielenz JR, Xiao X, Ge Y, Hamilton CY, Rodriguez M, Chen F, Foston M, Ragauskas A, Bouton J, Dixon RA, Wang ZY (2011) Genetic manipulation of lignin reduces recalcitrance and improves ethanol production from switchgrass. Proc Natl Acad Sci USA 108:3803–3808
Fu C, Sunkar R, Zhou C, Shen H, Zhang JY, Matts J, Wolf J, Mann DGJ, Stewart CN Jr, Tang Y, Wang ZY (2012) Overexpression of miR156 in switchgrass (Panicum virgatum L.) results in various morphological alterations and leads to improved biomass production. Plant Biotechnol J 10:443–452
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6:271–282
Kolesnik T, Szeverenyi I, Bachmann D, Kumar CS, Jiang S, Ramamoorthy R, Cai M, Ma ZG, Sundaresan V, Ramachandran S (2004) Establishing an efficient Ac/Ds tagging system in rice: large-scale analysis of Ds flanking sequences. Plant J 37:301–314
Kumar PP, Loh CS (2012) Plant tissue culture for biotechnology. In: Altman A, Hasegawa PM (eds) Plant biotechnology and agriculture. Academic Press, Amsterdam, pp 131–138
Li R, Qu R (2011) High throughput Agrobacterium-mediated switchgrass transformation. Biomass Bioenerg 35:1046–1054
Li L, Qu R, Kochko A, Fauquet C, Beachy RN (1993) An improved rice transformation system using the biolistic method. Plant Cell Rep 12:250–255
Mann DGJ, King ZR, Liu W, Joyce BL, Percifield RJ, Hawkins JS, LaFayette PR, Artelt BJ, Burris JN, Mazarei M, Bennetzen JL, Parrott WA, Stewart CN (2011) Switchgrass (Panicum virgatum L.) polyubiquitin gene (PvUbi1 and PvUbi2) promoters for use in plant transformation. BMC Biotechnol 11:74
Martinez-Reyna JM, Vogel KP (2002) Incompatibility systems in switchgrass. Crop Sci 42:1800–1805
McLaughlin S, Bouton J, Bransby D, Conger B, Ocumpaugh W, Parrish D, Taliaferro C, Vogel K, Wullschleger S (1999) Developing switchgrass as a bioenergy crop. In: Janick J (ed) Perspectives on new crops and new uses. ASHS Press, Alexandria, pp 282–299
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio-assay with tobacco tissue cultures. Physiol Plant 15:473–497
Peng J, Richards DE, Hartley NM, Murphy GP, Devos KM, Flintham JE, Beales J, Fish LJ, Worland AJ, Pelica F, Sudhakar D, Christou P, Snape JW, Gale MD, Harberd NP (1999) ‘Green revolution’ genes encode mutant gibberellin response modulators. Nature 400:256–261
Perrin R, Vogel K, Schmer M, Mitchell R (2008) Farm-scale production cost of switchgrass for biomass. BioEnerg Res 1:91–97
Schmer MR, Vogel KP, Mitchell RB, Perrin RK (2008) Net energy of cellulosic ethanol from switchgrass. Proc Natl Acad Sci USA 105:464–469
Shen H, He X, Poovaiah CR, Wuddineh WA, Ma J, Mann DGJ, Wang H, Jackson L, Tang Y, Neal Stewart C, Chen F, Dixon RA (2012) Functional characterization of the switchgrass (Panicum virgatum) R2R3-MYB transcription factor PvMYB4 for improvement of lignocellulosic feedstocks. New Phytol 193:121–136
Somleva MN, Tomaszewski Z, Conger BV (2002) Agrobacterium-mediated genetic transformation of switchgrass. Crop Sci 42:2080–2087
Vogel KP, Jung HG (2001) Genetic modification of herbaceous plants for feed and fuel. Crit Rev Plant Sci 20:15–49
Vogel KP, Gon HJ, HF A (1989) Breeding grasses for the future. In: Sleper DA, Asay KH, Pedersen JF (eds) Contributions from breeding forage and turf grasses. Crop Society of America, Madison, pp 105–122
Xi Y, Fu C, Ge Y, Nandakumar R, Hisano H, Bouton J, Wang Z-Y (2009) Agrobacterium-mediated transformation of switchgrass and inheritance of the transgenes. BioEnerg Res 2:275–283
Yin Z, Wang G-L (2000) Evidence of multiple complex patterns of T-DNA integration into the rice genome. Theor Appl Genet 100:461–470
Young HA, Hernlem BJ, Anderton AL, Lanzatella CL, Tobias CM (2010) Dihaploid stocks of switchgrass isolated by a screening approach. BioEnerg Res 3:305–313
Acknowledgments
This work was funded by the Science and Engineering Research Council (SERC Grant No.: 0921390036) of the Agency for Science Technology and Research, Singapore; and the National University of Singapore.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Lakshmanan.
Rights and permissions
About this article
Cite this article
Ramamoorthy, R., Kumar, P.P. A simplified protocol for genetic transformation of switchgrass (Panicum virgatum L.). Plant Cell Rep 31, 1923–1931 (2012). https://doi.org/10.1007/s00299-012-1305-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-012-1305-1