Abstract
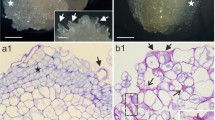
The study used Actinidia deliciosa endosperm-derived callus to investigate aspects of the morphology, histology and chemistry of extracellular matrix (ECM) structures in morphogenically stable tissue from long-term culture. SEM showed ECM as a membranous layer or reticulated fibrillar and granular structure linking the peripheral cells of callus domains. TEM confirmed that ECM is a distinct heterogeneous layer, up to 4 μm thick and consisting of amorphous dark-staining material, osmiophilic granules and reticulated fibres present outside the outer callus cell wall. ECM covered the surface of cells forming morphogenic domains and was reduced during organ growth. This structure may be linked to acquisition of morphogenic competence and thus may serve as a structural marker of it in endosperm-derived callus. ECM was also observed on senescent cells in contact with the morphogenic area. Treatment of living calluses with chloroform and washing with ether–methanol led to partial destruction of the extracellular layer. Digestion with pectinase removed the membranous layer almost completely and exposed thick fibrillar strands and granular remnants. Digestion with protease did not visibly affect the surface layer. Indirect immunofluorescence showed low-methylesterified pectic epitopes labelled by JIM5 monoclonal antibody. Immunolabelling, histochemistry, and solvent and enzyme treatments suggested pectins and lipids as components of the surface layer. These compounds may indicate protective, water retention and/or cell communication functions for this external layer.



Similar content being viewed by others
Abbreviations
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- BSA:
-
Bovine serum albumin
- CPD:
-
Critical point drying
- DAPI:
-
4′, 6-Diamidino-2-phenylindole dihydrochloride
- ECM:
-
Extracellular matrix
- ECMSN:
-
Extracellular matrix surface network
- EGTA:
-
Ethylene glycol-bis(β-aminoethyl ether)N, N, N′, N′-tetraacetic acid
- PBS:
-
Phosphate-buffered saline
- PIPES:
-
Piperazine-N, N′-bis(2-ethanesulfonic acid)
- SB:
-
Stabilising buffer
- SEM:
-
Scanning electron microscopy
- TEM:
-
Transmission electron microscopy
References
Baluška F, Parker JS, Barlow PW (1992) Specific patterns of cortical and endoplasmic microtubules associated with cell growth and tissue differentiation in roots of maize (Zea mays L.). J Cell Sci 103:191–200
Baluška F, Šamaj J, Wojtaszek P, Volkmann D, Menzel D (2003) Cytoskeleton–plasma membrane–cell wall continuum in plants. Emerging links revisited. Plant Physiol 133:482–491
Bobák M, Šamaj J, Hlinkova E, Hlavačka A, Ovečka M (2003/4) Extracellular matrix in early stages of direct somatic embryogenesis of Drosera spathulata. Biol Plant 47:161–162
Bobák M, Šamaj J, Pret’ová A, Blehová A, Hlinkova E, Ovečka M, Hlavačka A, Kutarňová (2004) The histological analysis of indirect somatic embryogenesis on Drosera spathulata Labill. Acta Physiol Plant 26:353–361
Chapman A, Helleboid S, Blervacq AS, Vasseur J, Hilbert JL (2000a) Removal of the fibrillar network surrounding Cichorium somatic embryos using cytoskeletal inhibitors: analysis of proteic components. Plant Sci 150:103–114
Chapman A, Blervacq AS, Tissier JP, Denbreil B, Vasseur J, Hilbert JL (2000b) Cell wall differentiation during early somatic embryogenesis in plants. I. Scanning and transmission electron microscopy originating from direct, indirect, and adventitious pathways. Can J Bot 78:816–823
Chapman A, Blervacq AS, Hendriks T, Slomianny, Vasseur J, Hilbert JL (2000c) Cell wall differentiation during early somatic embryogenesis in plants. II. Ultrastructural study and pectin immunolocalization on chicory embryos. Can J Bot 78:824–831
Dubois T, Guedira M, Dubois J, Vasseur J (1991) Direct somatic embryogenesis in leaves of Cichorium. A histological and SEM study of early stages. Protoplasma 162:120–127
Dubois T, Dubois J, Guedira M, Diop A, Vasseur J (1992) SEM characterization of an extracellular matrix around somatic proembryos in roots in Cichorium. Ann Bot 70:119–124
Dumville JC, Fry SC (2000) Uronic acid-containing oligosaccharins: their biosynthesis, degradation and signalling roles in non-diseased plant tissues. Plant Physiol Biochem 38:125–140
Fernando JA, Vieira MLC, Machado SR, Appezzato-da-Gloria B (2007) New insights into the in vitro organogenesis process: the case of Passiflora. Plant Cell Tiss Organ Cult 91:37–44
Góralski G, Popielarska M, Ślesak H, Siwińska D, Batycka M (2005) Organogenesis in endosperm of Actinidia deliciosa cv. Hayward cultured in vitro. Acta Biol Cracov Ser Bot 47:121–128
Guillemin F, Guillon F, Bonnin E, Devaux MF, Chevalier T, Knox JP, Liners F, Thibault JF (2005) Distribution of pectic epitopes in cell walls of the sugar beet root. Planta 222:355–371
Iwai H, Kikuchi A, Kobayashi T, Kamada H, Satoh S (1999) High levels of non-methylesterified pectins and low levels of peripherally located pectins in loosely attached non-embryogenic callus of carrot. Plant Cell Rep 18:561–566
Jarvis MC, Briggs SPH, Knox JP (2003) Intercellular adhesion and cell separation in plants. Plant Cell Environ 26:977–989
Kikuchi A, Satoh S, Nakamura N, Fujii T (1996) Differences in pectic polysaccharides between carrot embryogenic and non-embryogenic calli. Plant Cell Rep 14:279–284
Knox JP, Linstead PJ, Peart J, Cooper C, Roberts K (1990) Pectin esterification is spatially regulated both within cell walls and between developing tissues of root apices. Planta 181:512–521
Konieczny R, Bohdanowicz J, Czaplicki AZ, Przywara L (2005) Extracellular matrix surface network during plant regeneration in wheat anther culture. Plant Cell Tiss Organ Cult 83:201–208
Konieczny R, Świerczyńska J, Czaplicki AZ, Bohdanowicz J (2007) Distribution of pectin and arabinogalactan protein epitopes during organogenesis from androgenic callus of wheat. Plant Cell Rep 26:355–363
Liners F, Gaspar T, Van Cutsem P (1994) Acetyl- and methyl-esterification of pectins of friable and compact sugar-beet calli: consequences for intercellular adhesion. Planta 192:545–556
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15:473–497
Namasivayam P, Skepper J, Hanke D (2006) Identification of a potential structural marker for embryogenic competency in the Brassica napus spp. oleifera embryogenic tissue. Plant Cell Rep 25:887–895
Ovečka M, Bobák M (1999) Structural diversity of Papaver somniferum L. cell surfaces in vitro depending on particular steps of plant regeneration and morphogenetic program. Acta Physiol Plant 21:117–126
Popielarska M, Ślesak H, Góralski G (2006) Histological and SEM studies on organogenesis in endosperm-derived callus of kiwifruit (Actinidia deliciosa cv. Hayward). Acta Biol Cracov Ser Bot 48/2:97–104
Pret’ová A, Šamaj J, Obert B (2006) Cytological, physiological and biochemical aspects of somatic embryo formation in flax. In: Mujib A, Šamaj J (eds) Somatic embryogenesis. Springer, Berlin
Roberts K (1994) The plant extracellular matrix: in a new expansive mood. Curr Opin Cell Biol 6:688–694
Rumyansteva NI, Šamaj J, Ensikat HJ, Sal’nikov VV, Kostyukova YA, Baluška F, Volkmann D (2003) Changes in the extracellular matrix surface network during cyclic reproduction of proembryogenic cell complex in the Fagopyrum tataricum (L.) Gaertn callus. Dokl Biol Sci 391:375–378
Sondahl MR, Salisburi JL, Sharp WR (1979) SEM characterization of embryogenic tissue and globular embryos during high frequency somatic embryogenesis in coffee callus cells. Z Pflanzenphysiol 94:185–187
Šamaj J, Baluška F, Bobák M, Volkmann D (1999a) Extracellular matrix surface network of embryogenic units of friable maize callus contains arabinogalactan-proteins recognized by monoclonal antibody JIM4. Plant Cell Rep 18:369–374
Šamaj J, Ensikat HJ, Baluška F, Knox JP, Barthlott W, Volkmann D (1999b) Immunogold localization of plant surface arabinogalactan-proteins using glycerol liquid substitution and scanning electron microscopy. J Microscopy 193:150–157
Šamaj J, Bobák M, Blehová A, Pret’ová A (2006) Importance of cytoskeleton and cell wall in somatic embryogenesis. In: Mujib A, Šamaj J (eds) Somatic embryogenesis. Springer, Berlin
Šamaj J, Salaj T, Matúšová R, Salaj J, Takáč T, Šamajová O, Volkmann D (2007) Arabinogalactan-protein epitope Gal4 differentially regulated and localized in cell lines of hybrid fir (Abies alba × Abies cephalonica) with different embryogenic and regeneration potential. Plant Cell Rep doi:10.107/s00299-007-0429-1
Verdeil JL, Hocher V, Huet C, Grosdemange F, Escoute J, Ferrière N, Nicole M (2001) Ultrastructural changes in coconut calluses associated with the acquisition of embryogenic competence. Ann Bot 88:9–18
Willats WGT, McCartney L, Mackie W, Knox JP (2001) Pectin: cell biology and prospects for functional analysis. Plant Mol Biol 47:9–27
Acknowledgments
The authors are grateful to Prof. Dr. Elżbieta Kuta (Jagiellonian University) for critically reading the manuscript and making valuable suggestions. The SEM images were made in the Laboratory of Field Emission Scanning Electron Microscopy and Microanalysis at the Institute of Geological Sciences of the Jagiellonian University. We thank Jadwiga Faber for her expert technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by D. Somers.
Rights and permissions
About this article
Cite this article
Popielarska-Konieczna, M., Kozieradzka-Kiszkurno, M., Świerczyńska, J. et al. Ultrastructure and histochemical analysis of extracellular matrix surface network in kiwifruit endosperm-derived callus culture. Plant Cell Rep 27, 1137–1145 (2008). https://doi.org/10.1007/s00299-008-0534-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-008-0534-9