Abstract
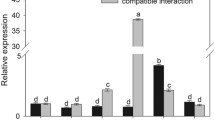
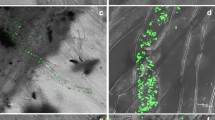
Metacaspases are cysteine proteinases that have homology to caspases, which play a central role in signaling and executing programmed cell death in animals. A type II metacaspase cDNA, NbMCA1, was amplified from Nicotiana benthamiana infected with Colletotrichum destructivum. It showed a peak in expression at 72 h post-inoculation corresponding with the switch to necrotrophy by C. destructivum. Inoculation of N. benthamiana with an incompatible bacterium, Pseudomonas syringae pv. tomato, which should induce a non-host hypersensitive response (HR), did not result in an increase in NbMCA1 expression at the time of necrosis development at 20–24 h postinoculation. Virus-induced silencing of NbMCA1 resulted in three to four times more lesions due to C. destructivum compared with leaves inoculated with the PVX vector without the cloned metacaspase gene or inoculated with water only. However, virus-induced silencing of NbMCA1 did not affect the HR necrosis or population levels of P. syringae pv. tomato. Although this metacaspase gene does not appear to be involved in the programmed cell death of non-host HR resistance to P. syringae, it does affect the susceptibility of N. benthamiana to C. destructivum indicating a function in a basal defense response. Possible roles of NbMCA1could be in degrading virulence factors of the pathogen, processing pro-proteins involved in stress responses, eliminating damaged proteins created during stress, and/or degrading proteins to remobilize amino acids to fuel de novo synthesis of proteins involved in stress adaptations.




Similar content being viewed by others
References
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3789–3402
Armando B, Julio M, Mario RS, Helena P (2003) Metacaspase related to programmed cell death in Arabidopsis thaliana. In: Proceedings of the 7th International Conference on Plant Molecular Biology, pp S21–72
Baulcombe D, Hamilton A, Voinnet O, Lu R, Peart JR, Malcuit I, Moffett P (2002) Sense and susceptibility: dissecting disease resistance using viruses and silencing. In: Leong SA, Allen C, Triplett EW (eds) Biology of plant–microbe interactions. International Society for Molecular Plant–Microbe Interactions, St. Paul, pp 1–10
Beers EP, Woffenden BJ, Zhao C (2000) Plant proteolytic enzymes: possible roles during programmed cell death. Plant Mol Biol 44:399–415
Bozhkov PV, Suarez MF, Filonova LH, Daniel G, Zamyatnin AA, Rodriguez-Nieto S, Zhivotovsky B, Smertenko A (2005) Cysteine protease mcll-Pa executes programmed cell death during plant embryogenesis. Proc Natl Acad Sci USA 102:14463–14468
Chen GYJ, Jin S, Goodwin PH (2000) An improved method for the isolation of total RNA from Malva pusilla tissues infected with Colletotrichum gloeosporioides. J Phytopathol 148:57–60
Chen N, Hsiang T, Goodwin PH (2002) Use of green fluorescent protein to quantify the growth of Colletotrichum during infection of tobacco. J Microbiol Methods 53:113–122
Cohen GM (1997) Caspases: the executioners of apoptosis. Biochem J 326:1–16
Cryns V, Yuan J (1998) Proteases to die for. Genes Dev 12:1551–1570
Dean JD, Goodwin PH, Hsiang T (2002) Comparison of relative RT-PCR and northern blot analyses to measure expression of β-1,3-glucanase in Nicotiana benthamiana infected with Colletotrichum destructivum. Plant Mol Biol Rep 20:347–356
del Pozo O, Lam E (1998) Caspases and programmed cell death in the hypersensitive response of plants to pathogens. Curr Biol 8:1129–1132
Dickman MB, Park YK, Oltersdorf T, Li W, Clemente T, French R (2001) Abrogation of disease development in plants expressing animal antiapoptotic genes. Proc Natl Acad Sci USA 98:6957–62
Espinosa A, Guo M, Tam VC, Fu ZQ, Alfano JR (2003) The Pseudomonas syringae type III-secreted protein HopPtoD2 possesses protein tyrosine phosphatase activity and suppresses programmed cell death in plants. Mol Microbiol 49:377–387
Forsthoefel NR, Cushman MAF, Ostrem JA, Cushman JC (1998) Induction of a cysteine protease cDNA from Mesembryanthemum crystallinum leaves by environmental stress and plant growth regulators. Plant Sci 136:195–206
Hao L, Hsiang T, Goodwin PH (2006) Role of two cysteine proteinases in the susceptible response of Nicotiana benthamiana to infection by Colletotrichum destructivum and the hypersensitive response to Pseudomonas syringae pv. tomato. Plant Sci 170:1001–1009
Hatsugai N, Kuroyanagi M, Yamada K, Meshi T, Tsuda S, Kondo M, Nishimura M, Hara-Nishimura I (2004) A plant vacuolar protease, VPE, mediates virus-induced hypersensitive cell death. Science 305:855–858
Heath MC (2000) Hypersensitive response-related death. Plant Mol Biol 44:321–334
Hedges SB (2002) The origin and evolution of model organisms. Nat Rev Genet 3:838–849
Hoeberichts FA, ten Have A, Woltering EJ (2003) A tomato metacaspase gene is upregulated during programmed cell death in Botrytis cinerea-infected leaves. Planta 217:517–522
Jones L, Hamilton AJ, Voinnet O, Thomas CL, Maule AJ, Baulcombe D (1999) RNA-DNA interactions and DNA methylation in post-transcriptional gene silencing. Plant Cell 11:2291–2301
King EO, Ward MK, Raney DE (1954) Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med 44:301–307
Klement Z (1963) Rapid detection of the pathogenicity of phytopathogenic Pseudomonads. Nature 199:299–300
Lam E, del Pozo DO (2000) Caspase-like protease involvement in the control of plant cell death. Plant Mol Biol 44:417–428
Lu R, Martin-Hernandez AM, Peart JR, Malcuit I, Baulcombe DC (2003) Virus-induced gene silencing in plants. Methods 30:296–303
Mandanhar J, Hartman G, Sinclair J (1986) Colletotrichum destructivum, the anamorph of Glomerella glycines. Phytopathology 76:282–285
Palma MJ, Sandalio ML, Javier CF, Romero-Puertas CM, McCathy I, del Rio AL (2002) Plant proteases, protein degradation, and oxidative stress: role of peroxisomes. Plant Physiol Biochem 40:521–530
Preston GM (2000) Pseudomonas syringae pv. tomato the right pathogen, of the right plant, at the right time. Mol Plant Pathol 1:263–275
Reed JC (2000) Mechanisms of apoptosis. Am J Pathol 157:1415–1430
Richael C, Lincoln JE, Bostock RM, Gilchrist DG (2001) Caspase inhibitors reduce symptom development and limit bacterial proliferation in susceptible plant tissues. Physiol Mol Plant Pathol 59:213–221
Robertson D (2004) VIGS vectors for gene silencing: many targets, many tools. Annu Rev Plant Biol 55:495–519
Rommens CMT, Salmeron JM, Oldroyd GED, Staskawicz BJ (1995) Intergeneric transfer and functional expression of the tomato disease resistance gene Pto. Plant Cell 7:1537–1544
Ruiz M, Voinnet O, Baulcombe D (1998) Initiation and maintenance of virus-induced gene silencing. Plant Cell 10: 937–946
Scofield SR, Tobias CM, Rathjen JP, Chang JH, Lavelle DT, Michelmore RW, Staskawicz BJ (1996) Molecular basis of gene-for-gene specificity in bacterial speck disease of tomato. Science 274:2063–2065
Sessa G, Martin GB (2000) Signal recognition and transduction mediated by the tomato Pto kinase: a paradigm of innate immunity in plants. Microbes Infect 2:1591–1597
Shen S, Goodwin PH, Hsiang T (2001) Hemibiotrophic infection and identity of the fungus, Colletotrichum destructivum, causing anthracnose of tobacco. Mycol Res 105:1340–1347
Shimada T, Hiraiwa N, Nishimura M, Hara-Nishimura I (1994) Vacuolar processing enzyme of soybean that converts proprotein to the corresponding mature forms. Plant Cell Physiol 35:713–718
Solomon M, Belenghi B, Delledonne M, Menachem E, Levine A (1999) The involvement of cysteine proteases and protease inhibitor genes in the regulation of programmed cell death in plants. Plant Cell 11:431–443
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 24:4876–4882
Takken LWF, Luderer R, Gabriels HEJS, Westerink N, Lu R, Joosten HAJM (2000) A functional cloning strategy, based on a binary PVX-expression vector, to isolate HR-inducing cDNAs of plant pathogens. Plant J 24:275–283
Uren AG, O’Rourke K, Aravind LA, Pisabarro MT, Seshagiri S, Koonin EV, Dixit VM (2000) Identification of paracaspases and metacaspases: two ancient families of caspase-like proteins, one of which plays a key role in MALT lymphoma. Mol Cell 6:961–967
van Baarlen P, Woltering EJ, Staats M, van Kan JAL (2007) Histochemical and genetic analysis of host and non-host interactions of Arabidopsis with three Botrytis species: an important role for cell death control. Mol Plant Pathol 8:41–54
Vercammen D, van de Cotte B, De Jaeger G, Eeckhout D, Casteels P, Vandepoele K, Vandenberghe I, Van Beeumen J, Inze D, Van Breusegem F (2004) Type II metacaspases Atmc4 and Atmc9 of Arabidopsis thaliana cleave substrates after arginine and lysine. J Biol Chem 279:45329–45336
Watanabe N, Lam E (2004) Recent advance in the study of caspase-like proteases and Bax inhibitor-1 in plants: their possible roles as regulator of programmed cell death. Mol Plant Pathol 5:65–70
Watanabe N, Lam E (2005) Two arabidopsis metacaspases AtMCP1b and AtMCP2b are arginine/lysine-specific cysteine proteases and activate apoptosis-like cell death in yeast. J Biol Chem 280:14691–14699
Acknowledgments
Funding for this study was provided by the Natural Science and Engineering Research Council of Canada. Pseudomonas syringae pv. tomato DC3000 was kindly provided by Dr. Dianne Cuppels, Agriculture and Agri-Food Canada, London, ON, Canada.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Judelson.
Rights and permissions
About this article
Cite this article
Hao, L., Goodwin, P.H. & Hsiang, T. Expression of a metacaspase gene of Nicotiana benthamiana after inoculation with Colletotrichum destructivum or Pseudomonas syringae pv. tomato, and the effect of silencing the gene on the host response. Plant Cell Rep 26, 1879–1888 (2007). https://doi.org/10.1007/s00299-007-0387-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-007-0387-7