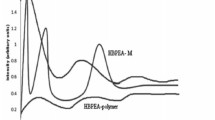
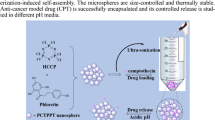
Abstract
Ibuprofen (IBU) is a non-steroidal anti-inflammatory drug (NSAID) with anti-inflammatory, analgesic, and antipyretic properties used to treat rheumatoid arthritis, osteoarthritis, and mild to moderate pain. However, it has extremely low aqueous solubility, rapid biotransformation with a half-life of nearly 2 h and poor tissue absorption resulting in poor bioavailability. One of the most important strategies for avoiding NSAID-related toxicity is modified release polymeric systems. Cross-linked polyphosphazene microspheres are attractive systems in drug delivery, as they are biocompatible and biodegradable. In the current work, novel cross-linked inorganic hybrid polyphosphazene microspheres were synthesized by self-assembly precipitation polymerization between hexachlorocyclotriphosphazene (trimer)/octachlorocyclotetraphosphazene (tetramer) as a cross-linker and 4,7-dihydroxyisoflavone (4.7 DHF) as a monomer. The structure of synthesized microspheres was identified several techniques in detail. IBU was successfully incorporated into the trimeric and tetrameric polyphosphazene microspheres. Particle size, zeta potential, drug loading, morphology, thermal, FT-IR, and XRD analyses, and IBU quantification using UPLC method were carried out to evaluate the microspheres. Considering the higher drug loading values and smaller particle size, trimer-based microspheres (4.7 DHF-TRI-IBU) were selected for further studies. In vitro release studies from 4.7 DHF-TRI-IBU demonstrated modified release pattern which followed zero-order kinetic model. Characteristics of 4.7 DHF-TRI-IBU were found to remain stable at 4 °C ± 1 °C, 25 °C ± 1 °C and 40 °C ± 1 °C during the storage period of 3 months. In vitro characterization analyses showed that spherical, micron-sized 4.7 DHF-TRI-IBU microspheres with prolonged release pattern has the potential of enhancing analgesic and anti-inflammatory activity.












Similar content being viewed by others
References
Qiu J, Wang Y, Liu Y, Zhang M, Wu Z, Liu C (2015) A pH-sensitive drug carrier based on maleic acid-substituted cyclotriphosphazene. Phosphorus Sulfur Silicon Relat Elem 190(9):1551–1561
Gleria M, De Jaeger R (2001) Aspects of phosphazene research. J Inorg Organomet Polym 11(1):1–45
Kumbar SG, Bhattacharyya S, Nukavarapu SP, Khan YM, Nair LS, Laurencin CT (2006) In vitro and in vivo characterization of biodegradable poly(organophosphazenes) for biomedical applications. J Inorg Organomet Polym Mater 16(4):365–385
Teasdale I, Brüggemann O (2013) Polyphosphazenes: multifunctional, biodegradable vehicles for drug and gene delivery. Polymers 5(1):161–187
Mark JE, Allcock HR, West R (2005) Inorganic polymers, 2nd edn. Oxford University Press, UK, pp 125–126
Zhang P, Huang X, Fu J, Huang Y, Zhu Y, Tang X (2009) A one-pot approach to novel cross-linked polyphosphazene microspheres with active amino groups. Macromol Chem Phys 210:792–798
Allcock HR, Fiztpatrick RJ, Salvati L (1991) Functionalization of the surface poly[bis(trifluoroethoxy)phosphazene] by reactions with alkoxide nucleophiles. Chem Mater 3:450–454
Duś D, Brandt K, Wojdat E, Gebarowska E, Jedliński Z, Radzikowski C (1989) Cytostatic activity in vitro of some new cyclophosphazene oligomers. Arch Immunol Ther Exp 37(5–6):539–546
Lee SB, Song SC, Jin J, Sohn YS (1999) Synthesis and antitumor activity of polyphosphazene/methoxy-poly(ethyleneglycol)/(diamine)platinum(II) conjugates. Polym J 31:1247–1252
Yıldırım T, Bilgin K, Çiftçi G, Tanriverdi Eçik E, Şenkuytu E, Uludağ Y, Tomak L, Kılıç A (2012) Synthesis, cytotoxicity and apoptosis of cyclotriphosphazene compounds as anti-cancer agents. Eur J Med Chem 52:213–220
Yaguchi A, Mori S, Kitayama M, Onda T, Kurahashi A, Ando H (1992) The characteristics of cyclic phosphazene and its applications for hard coatings. Thin Solid Films 216(1):123–125
Kim TH, Seong AY (2009) Copolymerization and contact lens application of HEMA-substituted polyphosphazene. J Korean Chem Soc 53(3):340–344
Dinarvand R, Dorkoosh F, Hamidi M, Moghadam SH (2004) Polymeric delivery systems for biopharmaceuticals. Biotechnol Genet Eng 21(1):147–182. https://doi.org/10.1080/02648725.2004.10648053
Shim S, Yang S, Choe S (2004) Mechanism of the formation of stable microspheres by precipitation copolymerization of styrene and divinilybenzene. J Polym Sci Part A: Polym Chem 42:3967–3974
Macha IJ, Ben-Nissan B, Vilchevskaya EN, Morozova AS, Abali BE, Müller WH, Rickert W (2019) Drug delivery from polymer-based nanopharmaceuticals - an experimental study complemented by simulations of selected diffusion processes. Front Bioeng Biotechnol 7(37):1–14
Badri W, Eddabra R, Fessi H, Elaissari A (2014) Biodegradable polymer based nanoparticles: dermal and transdermal drug delivery. J Colloid Sci Biotechnol 3:141–149
Jaimes-Aguirre L, Gibbens-Bandala BV, Morales-Avila E, Ocampo-García BE, Seyedeh-Fatemeh M, Amirhosein A (2016) Polymer-based drug delivery systems, development and pre-clinical status. Curr Pharm Des 22(19):2886–2903
Tan D, Yuan P, Annabi-Bergaya F, Yu H, Liu D, Liu H, He H (2013) Natural halloysite nanotubes as mesoporous carriers for the loading of ibuprofen. Microporous Mesoporous Mater 179:89–98
Carreras N, Acuña V, Martí M, Lis MJ (2013) Drug release system of ibuprofen in PCL-microspheres. Colloid Polym Sci 291:157–165
Shiyani B, Gattani S, Surana S (2008) Formulation and evaluation of bi-layer tablet of metoclopramide hydrochloride and ibuprofen. AAPS Pharm Sci Tech 9(3):818–827
Patel PJ, Bhalla S (2007) Pain management. In: Lee M, Desai A (eds) Gibaldi’s drug delivery systems in pharmaceutical care. American Society of Health-System Pharmacists, US, pp 457–473
Bushra R, Aslam N (2010) An overview of clinical pharmacology of ibuprofen. Oman Med J 25(3):155–161
Slattery JT, Levy G (1977) Effect of ibuprofen on protein binding of warfarin in human serum. J Pharm Sci 66(7):1060
Williams BS, Buvanendran A (2011) Nonopioid analgesics: NSAIDs, COX-2 inhibitors, and acetaminophen. In: Benzon HT, Liu SS, Cohen SP, Narouze S, Candido KD (eds) Essentials of pain medicine, 3rd edn. Elsevier Inc, Amsterdam, pp 130–139
Dweck AC (2006) Isoflavones, phytohormones and phytosterols. J Appl Cosmetol 24:17–33
Metinoğlu-Örüm S, Süzen-Demircioğlu Y (2018) Crosslinked polyphosphazene nanospheres with anticancer quercetin: synthesis, spectroscopic, thermal properties, and controlled drug release. Macromol Res 26:671–679
ICH Guideline. Q2 (R1):Validation of Analytical Procedure: Text and Methodology (2005), London. https://academy.gmp-compliance.org/guidemgr/files/Q2(R1).pdf. Accessed 15 June 2020
Srinivas P, Pragna S (2012) Formulation and evaluation of moxifloxacin hydrochloride ocular nanoparticles. Int J Nano Dimens 3(2):105–113
D’Souza SS, DeLuca PP (2006) Methods to assess in vitro drug release from injectable polymeric particulate systems. Pharm Res 23(3):460–474
Shen J, Burgess DJ (2013) In vitro dissolution testing strategies for nanoparticulate drug delivery systems: recent developments and challenges. Drug Deliv Transl Res 3(5):409–415
Yurtdaş-Kırımlıoğlu G, Menceloğlu Y, Erol K, Yazan Y (2016) In vitro/in vivo evaluation of gamma-aminobutyric acid-loaded N, N-dimethylacrylamide-based pegylated polymeric nanoparticles for brain delivery to treat epilepsy. J Microencapsul 33(7):625–635. https://doi.org/10.1080/02652048.2016.1234515
Fu J, Huang X, Zhu L, Tang X (2008) One-pot synthesis of porous cyclomatrix-type polyphosphazenenanotubes with closed ends via an in situ template approach. Scr Mater 58:1047–1049
Ozay H, Ozay O (2014) Synthesis and characterization of drug microspheres containing phosphazene for biomedical applications. Colloids Surf A Physicochem Eng Asp 450:99–155
Lopedota A, Trapani A, Cutrignelli A, Chiarantini L, Pantucci E, Curci R, Manuali E, Trapani G (2009) The use of eudragit® RS 100/cyclodextrin nanoparticles for the transmucosal administration of glutathione. Eur J Pharm and Biopharm 72(3):509–520
Honary S, Zahir F (2013) Effect of zeta potential on the properties of nano-drug delivery systems - a review (part 1). Trop J Pharm Res 12(2):255–264
Hou S, Chen S, Dong Y, Gao S, Zhu B, Lu Q (2018) Biodegradable cyclomatrix polyphosphazene nanoparticles: a novel ph-responsive drug self-framed delivery system. ACS Appl Mater Interfaces 10:25983–25993
Zheng C, Xu J, Yao X, Xu J, Qiu L (2011) Polyphosphazene nanoparticles for cytoplasmic release of doxorubicin with improved cytotoxicity against dox-resistant tumor cells. J Colloid Interf Sci 355:374–382
Süzen Y, Metinoğlu-Örüm S (2017) Novel cyclomatrix-type polyphosphazene microspheres crosslinked with octachlorocyclotetraphosphazene: preparation and characterization. Anadolu Univ J Sci Technol A: Appl Sci and Eng 18(5):973–987
Hu Y, Meng L, Niu L, Lu Q (2013) Highly cross-linked and biocompatible polyphosphazene-coated superparamagnetic Fe3O4 nanoparticles for magnetic resonance imaging. Langmuir 29(29):9156–9163
Wei W, Lu R, Ye W, Sun J, Zhu Y, Luo J, Liu X (2016) Liquid marbles stabilized by fluorine bearing cyclomatrix polyphosphazene particles and their application as high efficiency miniature reactors. Langmuir 32(7):1707–1715
Akbari B, Tavandashti MP, Zandrahimi M (2011) Particle size characterization of nanoparticles- a practical approach. Iran J Mater Sci and Eng 8(2):48–56
Panyam J, Labhasetwar V (2003) Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev 55(3):329–347
Mohanraj VJ, Chen Y (2006) Nanoparticles - a review. Trop J Pharm Res 5(1):561–573
De Campos AM, Sanchez A, Alonso MJ (2001) Chitosan nanoparticles: a new vehicle for the improvement of the delivery of drugs to the ocular surface. application to cyclosporin a. Int J Pharm 224:159–168. https://doi.org/10.1016/S0378-5173(01)00760-8
Katara R, Majumdar DK (2013) Eudragit RL 100-based nanoparticulate system of aceclofenac for ocular delivery. Colloid Surf B 103:455–462. https://doi.org/10.1016/j.colsurfb.2012.10.056
Sunkara G, Kompella UB (2003) Membrane transport processes in the eye. In: Mitra AK (ed) Ophthalmic drug delivery systems. Marcel Dekker Inc, New York, pp 13–58
Yurtdaş-Kırımlıoğlu G (2019) A systematic evaluation of formulation parameters on the characteristics of biodegradable PLGA-based nanoparticles for ophthalmic application. Lat Am J Pharm 38(11):2131–2142
Yurtdaş-Kırımlıoğlu G, Görgülü Ş, Berkman MS (2020) Novel approaches to cancer therapy with ibuprofen loaded Eudragit RS 100 and/or octadecylamine modified PLGA nanoparticles by assessment of their effects on apoptosis. Drug Dev Ind Pharm 46(7):1133–1149
Balaji RA, Raghunathan S, Revathy R (2015) Levofloxacin: formulation and in-vitro evaluation of alginate and chitosan nanoparticles. Egypt Pharm J 14(1):30–35
Caldorera-Moore M, Guimard N, Shi L, Roy K (2010) Designer nanoparticles: incorporating size, shape, and triggered release into nanoscale drug carriers. Expert Opin Drug Deliv 7(4):479–495
Albanase A, Tang PS, Chan WCW (2012) The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Eng 14:1–16
Yurtdaş-Kırımlıoğlu G, Özer S, Büyükköroğlu G, Yazan Y (2018) Formulation and in vitro evaluation of moxifloxacin hydrochloride-loaded polymeric nanoparticles. Lat Am J Pharm 37(9):1850–1862
Bannach G, Arcaro R, Ferroni DC, Siqueira A, Treu-Filho O, Ionashiro M, Schnitzler E (2010) Thermoanalytical study of some anti-inflammatory analgesic agents. J Therm Anal Calorim 102(1):163–170
Mainardes RM, Evangelista RC (2005) Praziquantel-loaded PLGA nanoparticles: preparation and characterization. J Microencapsul 22(1):13–24
Pagar K, Vavia P (2013) Rivastigmine-loaded l-lactide-depsipeptide polymeric nanoparticles: decisive formulation variable optimization. Sci Pharm 81(3):865–885
Shin SB, Cho HY, Kim DD, Choi HG, Lee YB (2010) Preparation and evaluation of tacrolimus-loaded nanoparticles for lymphatic delivery. Eur J Pharm Biopharm 74(2):164–171
Yurtdaş-Kırımlıoğlu G, Öztürk AA (2020) Levocetrizine dihydrochloride-loaded chitosan nanoparticles: formulation and in vitro evaluation. Turk J Pharm Sci 17(1):27–35
Kamari Y, Ghiaci M (2016) Preparation and characterization of ibuprofen/modified chitosan/TiO2 hybrid composite as a controlled drug-delivery system. Micropor Mesopor Mat 234:361–369. https://doi.org/10.1016/j.micromeso.2016.07.030
Salmoria GV, Sibilia F, Henschel VG, Fare S, Tanzi MC (2017) Structure and properties of polycaprolactone/ibuprofen rods prepared by melt extrusion for implantable drug delivery. Polym Bull 74:4973–4987. https://doi.org/10.1007/s00289-017-1999-x
Lu B, Lv X, Le Y (2019) Chitosan-modified PLGA nanoparticles for control-released drug delivery. Polymers 11(2):304. https://doi.org/10.3390/polym11020304
Zhang Y, Hou M, Zhou J, Zou A, Li W, Yao C, Xie S (2010) DDsolver: an add-in program for modeling and comparison of drug dissolution profiles. AAPS J 12(3):263–271
Muthu MS, Kulkarni SA, Raju A, Feng S-S (2012) Theranostic liposomes of TPGS coating for targeted co-delivery of docetaxel and quantum dots. Biomaterials 33:3494–3501
Lakshmi S, Katti DS, Laurencin CT (2003) Biodegradable polyphosphazenes for drug delivery applications. Adv Drug Deliv Rev 55:467–482
Kumar S, Singh RK, Prasad DN, Bhardwaj TR (2017) Synthesis and in vitro degradation studies of substituted poly(organophosphazenes) for drug delivery applications. J Drug Deliv Sci Tech 38:135–142
Allcock HR, Pucher SR, Scopelianos AG (1994) Poly[(amino acid ester)phosphazenes]: synthesis, crystallinity, and hydrolytic sensitive in solution and the solid state. Macromolecules 27:1071–1075
Song SC, Lee SB, Jin JI, Sohn YS (1999) A new class of biodegradable thermosensitive polymers. I. Synthesis and characterization of poly(organophosphazenes) with methoxy-poly(ethylene glycol) and amino acid esters as side groups. Macromolecules 32:2188–2193
Lee BH, Song SC (2004) Synthesis and characterization of biodegradable thermosensitive poly(organophosphazene) gels. Macromolecules 37:4533–4537
Chun C, Lee SM, Kim CW, Hong K-Y, Kim SY, Yang HK, Song S-C (2009) Doxorubicin–polyphosphazene conjugate hydrogels for locally controlled delivery of cancer therapeutics. Biomaterials 30:4752–4762
Baillargeon AL, Mequanint K (2014) Biodegradable Polyphosphazene Biomaterials For Tissue Engineering And Delivery Of Therapeutics. Biomed Res Int. Article ID 761373
Cho JK, Park JW, Song SC (2012) Injectable and biodegradable poly (organophosphazene) gel containing silibinin: its physicochemical properties and anticancer activity. J Pharm Sci 101(7):2382–2391
Cho JK, Kuh HJ, Song SC (2014) Injectable poly(organophosphazene) hydrogel system for effective paclitaxel and doxorubicin combination therapy. J Drug Target 22(8):761–767
Yurtdaş-Kırımlıoğlu G, Özer S, Büyükköroğlu G, Yazan Y (2020) Moxifloxacin hydrochloride-loaded eudragit® RL 100 and kollidon® sr based nanoparticles: formulation in vitro characterization and cytotoxicity. Comb Chem High Throughput Screen 23:1850. https://doi.org/10.2174/1386207323666200428091945
Zuo J, Gaoi Y, Bou-Chacra N, Löbenberg R (2014) Evaluation of the DDSolver Software Applications. Biomed Res Int Article ID 204925:1-9.
Dash S, Murthy PN, Nath L, Chowdhury P (2010) Kinetic modelling on drug release from controlled drug delivery systems. Acta Pol Pharm Drug Res 67(3):217–223
Yurtdaş-Kırımlıoğlu G, Görgülü Ş (2019) Design and characterization of montelukast sodium loaded Kollidon® SR nanoparticles and evaluation of release kinetics and cytotoxicity potential. Lat Am J Pharm 38(7):1350–1360
Kavaz D (2011) Nanopartiküller. Nanobülten, Aylık Nanoteknoloji ve Nanotıp Bilim Dergisi 12:12–19
Yurtdaş-Kırımlıoğlu G, Yazan Y, Erol K, Çengelli-Ünel Ç (2015) Gamma-aminobutyric acid loaded halloysite nanotubes and in vitro-in vivo evaluation for brain delivery. Int J Pharm 495:816–826
Acknowledgement
The authors would like to thank to Abdi İbrahim (İstanbul, Turkey) for supplying a gift sample of ibuprofen, Management of BİBAM (Anadolu University) and Eskişehir Technical University for providing an opportunity in performing SEM, XRD and BET analyses. This study was financed by Anadolu University Scientific Research Project Foundation (No: 1706S377).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yurtdaş-Kırımlıoğlu, G., Süzen-Demircioğlu, Y., Berkman, M.S. et al. Synthesis, spectroscopic, thermal properties, in vitro release, and stability studies of ibuprofen-loaded microspheres cross-linked with hexachlorocyclotriphosphazene/octachlorocyclotetraphosphazene. Polym. Bull. 78, 6221–6250 (2021). https://doi.org/10.1007/s00289-020-03422-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-020-03422-x