Abstract
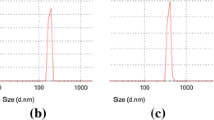
Chitosan nanoparticles (CSNPSs) were prepared from noncommercial chitosan of different molecular weights by the ionic gelation methodology. CSNPSs were applied in solid state (g) to observe the bacterial growth of Bacillus halotolerans MCC1, bacteria that appear in the biofilms of the desalinization membranes. Characterization of CSNPS of high (CSNP2), medium (CSNP3), and low (CSNP4) molecular weight (MW) was studied by TGA, DSC, SEM, EDX, DLS, and XRD. The effect of MW onto the formation of CSNPS was investigated as well as the effect of its application (0.01, 0.03, and 0.03 g at 24, 48, and 72 h) in the growth (%) of B. halotolerans MCC1. The results showed that the average size of CSNPS was in the range of 420–600.3 nm. CSNPS were considered amorphous and less thermally stable than the original chitosan where they were produced. Morphologically when the MW decreases, a reduction in the size of the particle was exhibited. When MW increase a reduction in the zeta potential from 45.7 to 29.9 mV was resulted. The essays to observe the growth (%) of B. halotolerans MCC1 indicated that CSNPS applied in solid state allows the growth of the bacteria; when the MW decreases, the growth of the bacteria increases. The maximum and minimum value of growth was obtained at 72 h, with the use of 0.05 g of CSNP4 (16.86%) and 0.05 g of CSNP2 (2.15%), respectively. The use of CSNPS indicates that the inhibitory effect depends on the MW as well as the doses of application.








Similar content being viewed by others
References
Kowalonek J (2017) Studies of chitosan/pectin complexes exposed to UV radiation. Int J Biol Macromol 103:515–524. https://doi.org/10.1016/j.ijbiomac.2017.05.081
Sun Z, Shi C, Wang X, Fang Q, Huang J (2017) Synthesis, characterization, and antimicrobial activities of sulfonated chitosan. Carbohydr Polym 155:321–328. https://doi.org/10.1016/j.carbpol.2016.08.069
Zeng D, Wu J, Kennedy JF (2008) Application of a chitosan flocculant to water treatment. Carbohydr Polym 71(1):135–139. https://doi.org/10.1016/j.carbpol.2007.07.039
Huang R, Liu Q, Huo J, Yang B (2017) Adsorption of methyl orange onto protonated cross-linked chitosan. Arab J Chem 10(1):24–32. https://doi.org/10.1016/j.arabjc.2013.05.017
Ahmed S, Ikram S (2016) Chitosan based scaffolds and their applications in wound healing. Achiev Life Sci 10(1):27–37. https://doi.org/10.1016/j.als.2016.04.001
Park BK, Kim M-M (2010) Applications of chitin and its derivatives in biological medicine. Int J Mol Sci. https://doi.org/10.3390/ijms11125152
Pant A, Negi JS (2018) Novel controlled ionic gelation strategy for chitosan nanoparticles preparation using TPP-β-CD inclusion complex. Eur J Pharm Sci 112:180–185. https://doi.org/10.1016/j.ejps.2017.11.020
Kim H, Lee E, Lee I-H, Lee J, Kim J, Kim S, Lee Y, Kim D, Choi M, Kim Y-C, Jon S (2014) Preparation and therapeutic evaluation of paclitaxel-conjugated low-molecular-weight chitosan nanoparticles. Macromol Res 22(8):805–808. https://doi.org/10.1007/s13233-014-2118-6
Rampino A, Borgogna M, Bellich B, Blasi P, Virgilio F, Cesàro A (2016) Chitosan-pectin hybrid nanoparticles prepared by coating and blending techniques. Eur J Pharm Sci 84:37–45. https://doi.org/10.1016/j.ejps.2016.01.004
Yang H-C, Hon M-H (2009) The effect of the molecular weight of chitosan nanoparticles and its application on drug delivery. Microchem J 92(1):87–91. https://doi.org/10.1016/j.microc.2009.02.001
Rinaudo M (2006) Chitin and chitosan: properties and applications. Prog Polym Sci 31(7):603–632. https://doi.org/10.1016/j.progpolymsci.2006.06.001
Hejjaji EMA, Smith AM, Morris GA (2018) Evaluation of the mucoadhesive properties of chitosan nanoparticles prepared using different chitosan to tripolyphosphate (CS:TPP) ratios. Int J Biol Macromol 120:1610–1617. https://doi.org/10.1016/j.ijbiomac.2018.09.185
Lallana E, Rios de la Rosa JM, Tirella A, Pelliccia M, Gennari A, Stratford IJ, Puri S, Ashford M, Tirelli N (2017) Chitosan/hyaluronic acid nanoparticles: rational design revisited for RNA delivery. Mol Pharm 14(7):2422–2436. https://doi.org/10.1021/acs.molpharmaceut.7b00320
Tsai L-C, Tsai M-L, Lu K-Y, Mi F-L (2018) Synthesis and evaluation of antibacterial and anti-oxidant activity of small molecular chitosan–fucoidan conjugate nanoparticles. Res Chem Intermed 44(8):4855–4871. https://doi.org/10.1007/s11164-018-3341-0
Sarwar A, Katas H, Zin NM (2014) Antibacterial effects of chitosan–tripolyphosphate nanoparticles: impact of particle size molecular weight. J Nanopart Res 16(7):2517. https://doi.org/10.1007/s11051-014-2517-9
Mohammadi A, Hashemi M, Masoud Hosseini S (2016) Effect of chitosan molecular weight as micro and nanoparticles on antibacterial activity against some soft rot pathogenic bacteria. LWT Food Sci Technol 71:347–355. https://doi.org/10.1016/j.lwt.2016.04.010
O'Callaghan KAM, Kerry JP (2016) Preparation of low- and medium-molecular weight chitosan nanoparticles and their antimicrobial evaluation against a panel of microorganisms, including cheese-derived cultures. Food Control 69:256–261. https://doi.org/10.1016/j.foodcont.2016.05.005
Ngan LTK, Wang S-L, Hiep ĐM, Luong PM, Vui NT, Đinh TM, Dzung NA (2014) Preparation of chitosan nanoparticles by spray drying, and their antibacterial activity. Res Chem Intermed 40(6):2165–2175. https://doi.org/10.1007/s11164-014-1594-9
Kim H-S, Lee JY, Ham S-Y, Lee JH, Park J-H, Park H-D (2019) Effect of biofilm inhibitor on biofouling resistance in RO processes. Fuel 253:823–832. https://doi.org/10.1016/j.fuel.2019.05.062
Romero-López GE, Alvarez-Sánchez J, de los Santos-Villalobos S, Fimbres-Weihs GA (2017) Biofouling studies on thin film composite membranes for reverse osmosis desalination processes. In: Maciel-Cerda A (ed) Membranes: materials, simulations, and applications. Springer, Cham, pp 99–104
Armendáriz-Ontiveros MM, García García A, de los Santos Villalobos S, Fimbres Weihs GA (2019) Biofouling performance of RO membranes coated with Iron NPs on graphene oxide. Desalination 451:45–58. https://doi.org/10.1016/j.desal.2018.07.005
Armendáriz-Ontiveros MM, Fimbres Weihs AG, de los Santos Villalobos S, Salinas-Rodriguez GS (2019) Biofouling of FeNP-coated SWRO membranes with bacteria isolated after pre-treatment in the sea of Cortez. Coatings. https://dx.doi.org/10.3390/coatings9070462
Sánchez-Duarte RG, Sánchez-Machado DI, López-Cervantes J, Correa-Murrieta MA (2012) Adsorption of allura red dye by cross-linked chitosan from shrimp waste. Water Sci Technol 65(4):618–623. https://doi.org/10.2166/wst.2012.900
Masuelli MA (2014) Mark–Houwink parameters for aqueous-soluble polymers and biopolymers at various temperatures. JPBPC 2(2):37–43. https://dx.doi.org/10.12691/jpbpc-2-2-2
Villegas-Peralta Y, Correa-Murrieta MA, Meza-Escalante ER, Flores-Aquino E, Álvarez-Sánchez J, Sánchez-Duarte RG (2019) Effect of the preparation method in the size of chitosan nanoparticles for the removal of allura red dye. Polym Bull 76(9):4415–4430. https://doi.org/10.1007/s00289-018-2601-x
Facchi SP, Scariot DB, Bueno PVA, Souza PR, Figueiredo LC, Follmann HDM, Nunes CS, Monteiro JP, Bonafé EG, Nakamura CV, Muniz EC, Martins AF (2016) Preparation and cytotoxicity of N-modified chitosan nanoparticles applied in curcumin delivery. Int J Biol Macromol 87:237–245. https://doi.org/10.1016/j.ijbiomac.2016.02.063
Thandapani G, Prasad P, Sudha PN, Sukumaran A (2017) Size optimization and in vitro biocompatibility studies of chitosan nanoparticles. Int J Biol Macromol 104:1794–1806. https://doi.org/10.1016/j.ijbiomac.2017.08.057
Dhawade PP, Jagtap RN (2012) Characterization of the glass transition temperature of chitosan and its oligomers by temperature modulated differential scanning calorimetry. Adv Appl Sci Res 3(3):1372–1382
Bhumkar DR, Pokharkar VB (2006) Studies on effect of pH on cross-linking of chitosan with sodium tripolyphosphate: a technical note. AAPS PharmSciTech 7(2):E50–E50. https://doi.org/10.1208/pt070250
Dananjaya SHS, Erandani WKCU, Kim C-H, Nikapitiya C, Lee J, De Zoysa M (2017) Comparative study on antifungal activities of chitosan nanoparticles and chitosan silver nano composites against Fusarium oxysporum species complex. Int J Biol Macromol 105:478–488. https://doi.org/10.1016/j.ijbiomac.2017.07.056
Kamel KM, Khalil IA, Rateb ME, Elgendy H, Elhawary S (2017) Chitosan-coated cinnamon/oregano-loaded solid lipid nanoparticles to augment 5-fluorouracil cytotoxicity for colorectal cancer: extract standardization, nanoparticle optimization, and cytotoxicity evaluation. J Agric Food Chem 65(36):7966–7981. https://doi.org/10.1021/acs.jafc.7b03093
Pereira AES, Silva PM, Oliveira JL, Oliveira HC, Fraceto LF (2017) Chitosan nanoparticles as carrier systems for the plant growth hormone gibberellic acid. Colloid Surface B 150:141–152. https://doi.org/10.1016/j.colsurfb.2016.11.027
Hajj Ali H, Michaux F, Khanji AN, Jasniewski J, Linder M (2018) Chitosan—Shea butter solid nanoparticles assemblies for the preparation of a novel nanoparticles in microparticles system containing curcumin. Colloids Surfaces A 553:359–367. https://doi.org/10.1016/j.colsurfa.2018.05.075
Hashad RA, Ishak RAH, Fahmy S, Mansour S, Geneidi AS (2016) Chitosan-tripolyphosphate nanoparticles: optimization of formulation parameters for improving process yield at a novel pH using artificial neural networks. Int J Biol Macromol 86:50–58. https://doi.org/10.1016/j.ijbiomac.2016.01.042
Ko JA, Park HJ, Hwang SJ, Park JB, Lee JS (2002) Preparation and characterization of chitosan microparticles intended for controlled drug delivery. Int J Pharm 249(1):165–174. https://doi.org/10.1016/S0378-5173(02)00487-8
Uppal S, Kaur K, Kumar R, Kaur ND, Shukla G, Mehta SK (2018) Chitosan nanoparticles as a biocompatible and efficient nanowagon for benzyl isothiocyanate. Int J Biol Macromol 115:18–28. https://doi.org/10.1016/j.ijbiomac.2018.04.036
MubarakAli D, LewisOscar F, Gopinath V, Alharbi NS, Alharbi SA, Thajuddin N (2018) An inhibitory action of chitosan nanoparticles against pathogenic bacteria and fungi and their potential applications as biocompatible antioxidants. Microb Pathog 114:323–327. https://doi.org/10.1016/j.micpath.2017.11.043
Ma J, Fu K, Shi J, Sun Y, Zhang X, Ding L (2016) Ultraviolet-assisted synthesis of polyacrylamide-grafted chitosan nanoparticles and flocculation performance. Carbohydr Polym 151:565–575. https://doi.org/10.1016/j.carbpol.2016.06.002
Rodríguez Hamamura N, Valderrama Negrón A, Alarcón Cavero H, López Milla A (2010) Preparación de partículas de quitosano reticuladas con tripolifosfato y modificadas con polietilenglicol. Rev Soc Quím Perú 76:336–354
Fan W, Yan W, Xu Z, Ni H (2012) Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids Surface B 90:21–27. https://doi.org/10.1016/j.colsurfb.2011.09.042
Fang N, Chan V, Mao H-Q, Leong KW (2001) Interactions of phospholipid bilayer with chitosan: effect of molecular weight and pH. Biomacromolecules 2(4):1161–1168. https://doi.org/10.1021/bm015548s
Gupta KC, Jabrail FH (2006) Effects of degree of deacetylation and cross-linking on physical characteristics, swelling and release behavior of chitosan microspheres. Carbohydr Polym 66(1):43–54. https://doi.org/10.1016/j.carbpol.2006.02.019
Maguire CM, Rösslein M, Wick P, Prina-Mello A (2018) Characterisation of particles in solution—a perspective on light scattering and comparative technologies. Sci Technol Adv Mat 19(1):732–745. https://doi.org/10.1080/14686996.2018.1517587
Taghizadeh MT, Bahadori A (2013) Preparation, characterization and adhesive properties of di- and tri-hydroxy benzoyl chitosan nanoparticles. Chin J Polym Sci 31(4):649–659. https://doi.org/10.1007/s10118-013-1247-2
Muthukrishnan S, Murugan I, Selvaraj M (2019) Chitosan nanoparticles loaded with thiamine stimulate growth and enhances protection against wilt disease in Chickpea. Carbohydr Polym 212:169–177. https://doi.org/10.1016/j.carbpol.2019.02.037
Li F, Jin H, Xiao J, Yin X, Liu X, Li D, Huang Q (2018) The simultaneous loading of catechin and quercetin on chitosan-based nanoparticles as effective antioxidant and antibacterial agent. Food Res Int 111:351–360. https://doi.org/10.1016/j.foodres.2018.05.038
Masarudin MJ, Cutts SM, Evison BJ, Phillips DR, Pigram PJ (2015) Factors determining the stability, size distribution, and cellular accumulation of small, monodisperse chitosan nanoparticles as candidate vectors for anticancer drug delivery: application to the passive encapsulation of [(14)C]-doxorubicin. Nanotechnol Sci Appl 8:67–80. https://doi.org/10.2147/nsa.s91785
Danaei M, Dehghankhold M, Ataei S, Hasanzadeh Davarani F, Javanmard R, Dokhani A, Khorasani S, Mozafari RM (2018) Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. https://doi.org/10.3390/pharmaceutics10020057
Subhapradha N, Shanmugam A (2017) Fabrication of β-chitosan nanoparticles and its anticancer potential against human hepatoma cells. Int J Biol Macromol 94:194–201. https://doi.org/10.1016/j.ijbiomac.2016.10.016
Hassani S, Laouini A, Fessi H, Charcosset C (2015) Preparation of chitosan–TPP nanoparticles using microengineered membranes—effect of parameters and encapsulation of tacrine. Colloid Surface A 482:34–43. https://doi.org/10.1016/j.colsurfa.2015.04.006
Gupta V, Trivedi P (2018) In vitro and in vivo characterization of pharmaceutical topical nanocarriers containing anticancer drugs for skin cancer treatment. In: Grumezescu AM (ed) Lipid nanocarriers for drug targeting. William Andrew Publishing, New York, pp 563–627
Horie M, Fujita K (2011) Toxicity of metal oxides nanoparticles. In: Fishbein JC (ed) Advances in molecular toxicology. Elsevier, Berlin, pp 145–178
Li Q, Liu C-G, Yu Y (2015) Separation of monodisperse alginate nanoparticles and effect of particle size on transport of vitamin E. Carbohydr Polym 124:274–279. https://doi.org/10.1016/j.carbpol.2015.02.007
Khan MA, Zafaryab M, Mehdi SH, Quadri J, Rizvi MMA (2017) Characterization and carboplatin loaded chitosan nanoparticles for the chemotherapy against breast cancer in vitro studies. Int J Biol Macromol 97:115–122. https://doi.org/10.1016/j.ijbiomac.2016.12.090
Du Z, Liu J, Zhang T, Yu Y, Zhang Y, Zhai J, Huang H, Wei S, Ding L, Liu B (2019) A study on the preparation of chitosan-tripolyphosphate nanoparticles and its entrapment mechanism for egg white derived peptides. Food Chem 286:530–536. https://doi.org/10.1016/j.foodchem.2019.02.012
Gonil P, Sajomsang W, Ruktanonchai UR, Na Ubol P, Treetong A, Opanasopit P, Puttipipatkhachorn S (2014) Synthesis and fluorescence properties of N-substituted 1-cyanobenz[f]isoindole chitosan polymers and nanoparticles for live cell imaging. Biomacromolecules 15(8):2879–2888. https://doi.org/10.1021/bm5004459
Fan Y, Yi J, Zhang Y, Yokoyama W (2017) Improved chemical stability and antiproliferative activities of curcumin-loaded nanoparticles with a chitosan chlorogenic acid conjugate. J Agric Food Chem. https://doi.org/10.1021/acs.jafc.7b04451
Yancheva E, Paneva D, Maximova V, Mespouille L, Dubois P, Manolova N, Rashkov I (2007) Polyelectrolyte complexes between (cross-linked) N-carboxyethylchitosan and (quaternized) poly[2-(dimethylamino)ethyl methacrylate]: preparation, characterization, and antibacterial properties. Biomacromolecules 8(3):976–984. https://doi.org/10.1021/bm061029j
Kingkaew J, Kirdponpattara S, Sanchavanakit N, Pavasant P, Phisalaphong M (2014) Effect of molecular weight of chitosan on antimicrobial properties and tissue compatibility of chitosan-impregnated bacterial cellulose films. Biotechnol Bioproc E 19(3):534–544. https://doi.org/10.1007/s12257-014-0081-x
Rodrı́guez H, Fraga R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv 17(4):319–339. https://doi.org/10.1016/S0734-9750(99)00014-2
Fernandes JC, Tavaria FK, Soares JC, Ramos ÓS, João Monteiro M, Pintado ME, Xavier Malcata F (2008) Antimicrobial effects of chitosans and chitooligosaccharides, upon Staphylococcus aureus and Escherichia coli, in food model systems. Food Microbiol 25(7):922–928. https://doi.org/10.1016/j.fm.2008.05.003
Madureira AR, Pereira A, Castro PM, Pintado M (2015) Production of antimicrobial chitosan nanoparticles against food pathogens. J Food Eng 167:210–216. https://doi.org/10.1016/j.jfoodeng.2015.06.010
Costa EM, Silva S, Vicente S, Neto C, Castro PM, Veiga M, Madureira R, Tavaria F, Pintado MM (2017) Chitosan nanoparticles as alternative anti-staphylococci agents: bactericidal, antibiofilm and antiadhesive effects. Mater Sci Eng C 79:221–226. https://doi.org/10.1016/j.msec.2017.05.047
Nguyen TV, Nguyen TTH, Wang S-L, Vo TPK, Nguyen AD (2017) Preparation of chitosan nanoparticles by TPP ionic gelation combined with spray drying, and the antibacterial activity of chitosan nanoparticles and a chitosan nanoparticle–amoxicillin complex. Res Chem Intermed 43(6):3527–3537. https://doi.org/10.1007/s11164-016-2428-8
No HK, Young Park N, Ho Lee S, Meyers SP (2002) Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int J Food Microbiol 74(1):65–72. https://doi.org/10.1016/S0168-1605(01)00717-6
Acknowledgements
The authors are grateful for the support of Laboratorio Nacional de Nano y Biomateriales (LANNBIO) at Centro de Investigación y de Estudios Avanzados (CINVESTAV) Mérida, Yucatán, México. The authors are grateful for the financial support Project PROFAPI_2019_0179 from Instituto Tecnologico de Sonora. The first author is grateful to CONACYT (269728).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Villegas-Peralta, Y., López-Cervantes, J., Madera Santana, T.J. et al. Impact of the molecular weight on the size of chitosan nanoparticles: characterization and its solid-state application. Polym. Bull. 78, 813–832 (2021). https://doi.org/10.1007/s00289-020-03139-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-020-03139-x