Abstract
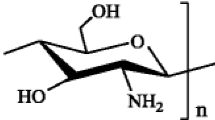
Chitosan–tripolyphosphate beads are a highly stable and biocompatible polymer-network with versatile applications. In this study, we relate the main synthesis parameters of these particles using a design of experiments and a multivariate calibration to understand how some structural characteristics (mass, diameter and water retention capacity) of these spherical hydrogels are achieved from the point of view of the ionotropic association between chitosan chains and tripolyphosphate molecules. The results conduct to a better conceptualization of the crosslinking process, establish the ranges where particles with different structural characteristic can be obtained and a better understanding of how the physicochemical behavior of the chitosan and the tripolyphosphate affect the structural parameters of the chitosan–tripolyphosphate beads. As a novelty, we propose a refined 3D junction zone for the interaction between the raw molecules that allows to conclude that one TPP molecule can associate with up to three amino groups as a maximum.









Similar content being viewed by others
References
Kašpar O, Jakubec M, Štěpánek F (2013) Characterization of spray dried chitosan–TPP microparticles formed by two- and three-fluid nozzles. Powder Technol 240:31–40. https://doi.org/10.1016/j.powtec.2012.07.010
Tokárová V, Kašpar O, Knejzlík Z et al (2013) Development of spray-dried chitosan microcarriers for nanoparticle delivery. Powder Technol 235:797–805. https://doi.org/10.1016/j.powtec.2012.12.005
Yuan D, Jacquier JC, O’Riordan ED (2017) Entrapment of protein in chitosan–tripolyphosphate beads and its release in an in vitro digestive model. Food Chem 229:495–501. https://doi.org/10.1016/j.foodchem.2017.02.107
Sun P, Li P, Li YM et al (2011) A pH-sensitive chitosan–tripolyphosphate hydrogel beads for controlled glipizide delivery. J Biomed Mater Res Part B Appl Biomater 97B:175–183. https://doi.org/10.1002/jbm.b.31801
Alsarra IA, Betigeri SS, Zhang H et al (2002) Molecular weight and degree of deacetylation effects on lipase-loaded chitosan bead characteristics. Biomaterials 23:3637–3644. https://doi.org/10.1016/S0142-9612(02)00096-0
Alsarra IA, Neau SH, Howard MA (2004) Effects of preparative parameters on the properties of chitosan hydrogel beads containing Candida rugosa lipase. Biomaterials 25:2645–2655. https://doi.org/10.1016/j.biomaterials.2003.09.051
Giraldo JD, Rivas BL, Elgueta E, Mancisidor A (2017) Metal ion sorption by chitosan–tripolyphosphate beads. J Appl Polym Sci 134:45511. https://doi.org/10.1002/app.45511
Dash M, Chiellini F, Ottenbrite RM, Chiellini E (2011) Chitosan—a versatile semi-synthetic polymer in biomedical applications. Prog Polym Sci 36:981–1014. https://doi.org/10.1016/j.progpolymsci.2011.02.001
Schrödter K, Bettermann G, Staffel T et al (2008) Phosphoric acid and phosphates. Ullmann’s Encycl. Ind. Chem. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, pp 679–724
Durkut S, Elçin YM, Elçin AE (2006) Biodegradation of chitosan–tripolyphosphate beads. In vitro and in vivo studies. Artif Cells Blood Substit Biotechnol 34:263–276. https://doi.org/10.1080/10731190600581866
Yadav SK, Khan G, Bansal M et al (2017) Screening of ionically crosslinked chitosan–tripolyphosphate microspheres using Plackett–Burman factorial design for the treatment of intrapocket infections. Drug Dev Ind Pharm 43:1801–1816. https://doi.org/10.1080/03639045.2017.1349782
Filion D, Lavertu M, Buschmann MD (2007) Ionization and solubility of chitosan solutions related to thermosensitive chitosan/glycerol-phosphate systems ionization and solubility of chitosan solutions related to thermosensitive chitosan/glycerol-phosphate systems. Biomacromolecules 8:3224–3234. https://doi.org/10.1021/bm700520m
Shu XZ, Zhu KJ (2002) The influence of multivalent phosphate structure on the properties of ionically cross-linked chitosan films for controlled drug release. Eur J Pharm Biopharm 54:235–243
Goycoolea FM, Brunel F, Gueddari NEE et al (2016) Physical properties and stability of soft gelled chitosan-based nanoparticles. Macromol Biosci 16:1873–1882. https://doi.org/10.1002/mabi.201600298
Mazancová P, Némethová V, Treľová D et al (2018) Dissociation of chitosan/tripolyphosphate complexes into separate components upon pH elevation. Carbohydr Polym 192:104–110. https://doi.org/10.1016/j.carbpol.2018.03.030
Joye IJ, McClements DJ (2014) Biopolymer-based nanoparticles and microparticles: fabrication, characterization, and application. Curr Opin Colloid Interface Sci 19:417–427. https://doi.org/10.1016/j.cocis.2014.07.002
Bjørnøy SH, Mandaric S, Bassett DC et al (2016) Gelling kinetics and in situ mineralization of alginate hydrogels: a correlative spatiotemporal characterization toolbox. Acta Biomater 44:243–253. https://doi.org/10.1016/j.actbio.2016.07.046
Huang Y, Lapitsky Y (2012) Salt-assisted mechanistic analysis of chitosan/tripolyphosphate micro- and nanogel formation. Biomacromolecules 13:3868–3876
Martins AF, de Oliveira DM, Pereira AGB et al (2012) Chitosan/TPP microparticles obtained by microemulsion method applied in controlled release of heparin. Int J Biol Macromol 51:1127–1133. https://doi.org/10.1016/j.ijbiomac.2012.08.032
Fwu-Long M, Shyu SS, Lee ST, Wong TBI (1999) Kinetic study of chitosan–tripolyphosphate complex reaction and acid-resistive properties of the chitosan–tripolyphosphate gel beads prepared by in-liquid curing method. J Polym Sci Part B Polym Phys 37:1551–1564. https://doi.org/10.1002/(SICI)1099-0488(19990715)37:14%3c1551:AID-POLB1%3e3.0.CO;2-H
Perez JJ, Francois NJ (2016) Chitosan-starch beads prepared by ionotropic gelation as potential matrices for controlled release of fertilizers. Carbohydr Polym 148:134–142. https://doi.org/10.1016/j.carbpol.2016.04.054
Giraldo JD, Rivas BL (2017) Determination of urea using p-N, N-dimethylaminobenzaldehyde: solvent effect and interference of chitosan. J Chil Chem Soc 2:3538–3542
Rinaudo M, Pavlov G, Desbrières J (1999) Influence of acetic acid concentration on the solubilization of chitosan. Polymer (Guildf) 40:7029–7032. https://doi.org/10.1016/S0032-3861(99)00056-7
Brugnerotto J, Lizardi J, Goycoolea FM et al (2001) An infrared investigation in relation with chitin and chitosan characterization. Polymer (Guildf) 42:3569–3580. https://doi.org/10.1016/S0032-3861(00)00713-8
Corbridge DEC (1995) Studies in inorganic chemistry 20-phosphorus—an outline of its chemistry, biochemistry an uses, 5th edn. Elsevier B.V., Amsterdam
de Levie R (1993) Explicit expressions of the general form of the titration curve in terms of concentration. J Chem Educ 70:209–217. https://doi.org/10.1021/ed070p209
Dean JA (1999) Lange’s handbook of chemistry, 15th edn. Mcgraw-Hill, New York. https://doi.org/10.1080/10426919008953291
Reichardt C, Welton T (2011) Solvents and solvents effects in organic chemistry, 4th edn. WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
Mahaninia MH, Wilson LD (2017) Phosphate uptake studies of cross-linked chitosan bead materials. J Colloid Interface Sci 485:201–212. https://doi.org/10.1016/j.jcis.2016.09.031
Mark D, Haeberle S, Zengerle R et al (2009) Manufacture of chitosan microbeads using centrifugally driven flow of gel-forming solutions through a polymeric micronozzle. J Colloid Interface Sci 336:634–641. https://doi.org/10.1016/j.jcis.2009.04.029
Fu J, Schlenoff JB (2016) Driving forces for oppositely charged polyion association in aqueous solutions: enthalpic, entropic, but not electrostatic. J Am Chem Soc 138:980–990. https://doi.org/10.1021/jacs.5b11878
Domard A (2011) A perspective on 30 years research on chitin and chitosan. Carbohydr Polym 84:696–703. https://doi.org/10.1016/j.carbpol.2010.04.083
Fan W, Yan W, Xu Z, Ni H (2012) Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids Surf B Biointerfaces 90:21–27. https://doi.org/10.1016/j.colsurfb.2011.09.042
Huang Y, Lapitsky Y (2011) Monovalent salt enhances colloidal stability during the formation of chitosan/tripolyphosphate microgels. Langmuir 27:10392–10399. https://doi.org/10.1021/la201194a
Yu SH, Wu SJ, Wu JY et al (2013) Tripolyphosphate cross-linked macromolecular composites for the growth of shape- and size-controlled apatites. Molecules 18:27–40. https://doi.org/10.3390/molecules18010027
Kleine-Brueggeney H, Zorzi GK, Fecker T et al (2015) A rational approach towards the design of chitosan-based nanoparticles obtained by ionotropic gelation. Colloids Surf B Biointerfaces 135:99–108. https://doi.org/10.1016/j.colsurfb.2015.07.016
Johansson E, Kettaneh-Wold N, Wikstrom C, Wold S, Ericksson L et al (2000) Design of experiments: principles and applications, 3rd edn
Ogawa K, Yui T, Okuyama K (2004) Three D structures of chitosan. Int J Biol Macromol 34:1–8. https://doi.org/10.1016/j.ijbiomac.2003.11.002
Okuyama K, Osawa K, Hanafusa Y et al (2000) Communication: relaxed 2/1-helical conformation of type II chitosan has a tetrasaccharide motif. J Carbohydr Chem 19:789–794. https://doi.org/10.1080/07328300008544117
Lertworasirikul A, Tsue SI, Noguchi K et al (2003) Two different molecular conformations found in chitosan type II salts. Carbohydr Res 338:1229–1233. https://doi.org/10.1016/S0008-6215(03)00145-9
Naito PK, Ogawa Y, Sawada D et al (2016) X-ray crystal structure of anhydrous chitosan at atomic resolution. Biopolymers 105:361–368. https://doi.org/10.1002/bip.22818
Franca EF, Lins RD, Freitas LCG, Straatsma TP (2008) Characterization of chitin and chitosan molecular structure in aqueous solution. J Chem Theory Comput 4:2141–2149. https://doi.org/10.1021/ct8002964
Lertworasirikul A, Noguchi K, Ogawa K, Okuyama K (2004) Plausible molecular and crystal structures of chitosan/HI type II salt. Carbohydr Res 339:835–843. https://doi.org/10.1016/j.carres.2004.01.002
Okuyama K, Noguchi K, Kanenari M et al (2000) Structural diversity of chitosan and its complexes. Carbohydr Polym 41:237–248. https://doi.org/10.1016/S0144-8617(99)00142-3
Kohn R (1975) Ion binding on polyuronates-alginate and pecitin. Pure Appl Chem 42:371–397. https://doi.org/10.1351/pac197542030371
Donati I, Holtan S, Mørch YA et al (2005) New hypothesis on the role of alternating sequences in calcium-alginate gels. Biomacromolecules 6:1031–1040. https://doi.org/10.1021/bm049306e
Donati I, Cesàro A, Paoletti S (2006) Specific interactions versus counterion condensation. 1. Nongelling ions/polyuronate systems. Biomacromolecules 7:281–287. https://doi.org/10.1021/bm050646p
Sacco P, Paoletti S, Cok M et al (2016) Insight into the ionotropic gelation of chitosan using tripolyphosphate and pyrophosphate as cross-linkers. Int J Biol Macromol 92:476–483. https://doi.org/10.1016/j.ijbiomac.2016.07.056
Mi FL, Shyu SS, Kuan CY et al (1999) Chitosan-polyelectrolyte complexation for the preparation of gel beads and controlled release of anticancer drug. I. Effect of phosphorous polyelectrolyte complex and enzymic hydrolysis of polymer. J Appl Polym Sci 74:1868–1879
Mi F-L, Shyu S-S, Wong T-B et al (1999) Chitosan-polyelectrolyte complexation for the preparation of gel beads and controlled release of anticancer drug. II. Effect of pH-dependent ionic crosslinking or interpolymer complex using tripolyphosphate or polyphosphate as reagent. J Appl Polym Sci 74:1093–1107. https://doi.org/10.1002/(SICI)1097-4628(19991031)74:5%3c1093:AID-APP6%3e3.0.CO;2-C
Huang Y, Lapitsky Y (2013) Determining the colloidal behavior of ionically cross-linked polyelectrolytes with isothermal titration calorimetry. J Phys Chem B 117:9548–9557. https://doi.org/10.1021/jp405384b
Yang CH, Huang KS, Lin PW, Lin YC (2007) Using a cross-flow microfluidic chip and external crosslinking reaction for monodisperse TPP-chitosan microparticles. Sens Actuators B Chem 124:510–516. https://doi.org/10.1016/j.snb.2007.01.015
Koukaras EN, Papadimitriou SA, Bikiaris DN, Froudakis GE (2012) Insight on the formation of chitosan nanoparticles through ionotropic gelation with tripolyphosphate. Mol Pharm 9:2856–2862. https://doi.org/10.1021/mp300162j
Kumirska J, Czerwicka M, Kaczyński Z et al (2010) Application of spectroscopic methods for structural analysis of chitin and chitosan. Mar Drugs 8:1567–1636. https://doi.org/10.3390/md8051567
Grubb DT, Keller A, Bassett DC et al (1988) Developments in crystalline polymers-2. https://doi.org/10.1007/978-94-009-1341-7
Donati I, Benegas JC, Cesàro A, Paoletti S (2006) Specific interactions versus counterion condensation. 2. Theoretical treatment within the counterion condensation theory. Biomacromolecules 7:1587–1596. https://doi.org/10.1021/bm050981d
Sarkhel S, Desiraju GR (2004) N–H···O, O–H···O, and C–H···O hydrogen bonds in protein-ligand complexes: strong and weak interactions in molecular recognition. Proteins 54:247–259. https://doi.org/10.1002/prot.10567
Wilson CC (2001) Migration of the proton in the strong O–H···O hydrogen bond in urea-phosphoric acid (1/1). Acta Crystallogr Sect B Struct Sci 57:435–439. https://doi.org/10.1107/S0108768100018875
Lawrie G, Keen I, Drew B et al (2007) Interactions between alginate and chitosan biopolymers characterized using FTIR and XPS. Biomacromolecules 8:2533–2541. https://doi.org/10.1021/bm070014y
Koukaras EN, Papadimitriou SA, Bikiaris DN, Froudakis GE (2014) Properties and energetics for design and characterization of chitosan nanoparticles used for drug encapsulation. RSC Adv 4:12653–12661. https://doi.org/10.1039/C3RA47572G
Desiraju G, Steiner T (2001) The weak hydrogen bond. Oxford University Press, New York. https://doi.org/10.1093/acprof:oso/9780198509707.001.0001
Acknowledgements
This study was realized with funds granted by CONICYT Chile scholarship No 63140018.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Giraldo, J.D., Campos-Requena, V.H. & Rivas, B.L. Chitosan–tripolyphosphate bead: the interactions that govern its formation. Polym. Bull. 76, 3879–3903 (2019). https://doi.org/10.1007/s00289-018-2574-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-018-2574-9