Abstract
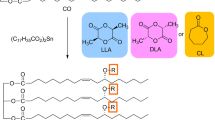
The reactions of diglycerol-based 4-armed enantiomeric lactide oligomers (DG4LLAO and DG4DLAO, DGDLAO/DGLLAO = 1/1) and a 1,3-propanediol-based 2-armed rac-lactide oligomer (PD2racLAO) with hexamethylene diisocyanate produced bio-based polyesterurethane networks (PEU-DG4scLAO/PD2racLAOs) with different feed ratios of stereocomplex (sc) lactide oligomer (DG4scLAO = DG4DLAO + DG4LLAO) and PD2racLAO. Crystallization behavior and physical properties of PEU-DG4scLAO/PD2racLAOs were compared with those of the corresponding pentaerythritol- and glycerol-based polyesterurethane networks (PEU-PE4scLAO and PEU-GC4scLAO) with no PD2racLAO fraction. The X-ray diffraction analysis revealed that sc crystallites were formed without any homo-crystallization for PEU-DG4scLAO/PD2racLAOs 100/0-25/75 in a similar manner to PEU-PE4scLAO or PEU-GC3scLAO. Differential scanning calorimetric analysis for PEU-DG4scLAO/PD2racLAOs 100/0-25/75 revealed that the sc crystallites were not regenerated during a cold crystallization process of the quenched samples, but regenerated by isothermal crystallization from the melt. This result was a marked contrast to the previous result that sc crystallites were almost completely regenerated by the cold crystallization for PEU-PE4scLAO and PEU-GC3scLAO. Polarized optical microscopic analysis revealed that the incorporation of 25% of PD2racLAO enhanced the sc-nucleation efficiency, and further addition caused the reduction of overall crystallization. PEU-DG4scLAO/PD2racLAO 100/0 exhibited a higher elongation at break and tensile toughness than PEU-PE4scLAO and PEU-GC3scLAO. Tensile strength and elongation at break for PEU-DG4scLAO/PD2racLAOs decreased with increasing feed of PD2racLAO.










Similar content being viewed by others
References
Iwata T (2015) Biodegradable and bio-based polymers: future prospects of eco-friendly plastics. Angew Chem Int Ed 54:3210–3215
Yao K, Tang C (2013) Controlled polymerization of next-generation renewable monomers and beyond. Macromolecules 46:1689–1712
Babu RP, O’Connor K, Seeram R (2013) Current progress on bio-based polymers and their future trends. Prog Biomater 2:8. doi:10.1186/2194-0517-2-8
Wilbon PA, Chu F, Tang C (2013) Progress in renewable polymers from natural terpenes, terpenoids, and rosin. Macromol Rapid Commun 34:8–37
Farah S, Anderson DG, Langer R (2016) Physical and mechanical properties of PLA, and their functions in widespread applications—a comprehensive review. Adv Drug Deliver Rev. doi:10.1016/j.addr.2016.012
Raquez JM, Habibi Y, Murariu M, Dubois P (2013) Polylactide (PLA)-based nanocomposites. Prog Polym Sci 38:1504–1542
Yi Q, Wen X, Li L, He B, Nie Y, Wu Y, Zhang Z, Gu Z (2009) The chiral effects on the responses of osteoblastic cells to the polymeric substrates. Eur Polym J 45:1970–1978
Tsuji H (2005) Poly(lactide) stereocomplexes: formation, structure, properties, degradation, and applications. Macromol Biosci 5:569–597
Tsuji H (2016) Poly(lactic acid) stereocomplexes: a decade of progress. Adv Drug Deliver Rev. doi:10.1016/j.addr.2016.04.017
Ikada Y, Jamshidi K, Tsuji H, Hyon SH (1987) Stereocomplex formation between enantiomeric poly(lactides). Macromolecules 20:904–906
Tsuji H, Ikada Y (1999) Stereocomplex formation between enantiomeric poly(lactic acid)s. XI. Mechanical properties and morphology of solution-cast films. Polymer 40:6699–6708
Tsuji H, Fukui I (2003) Enhanced thermal stability of poly(lactide)s in the melt by enantiomeric polymer blending. Polymer 44:2891–2896
Tsuji H (2000) In vitro hydrolysis of blends from enantiomeric poly(lactide)s Part 1. Well-stereo-complexed blend and non-blended films. Polymer 41:3621–3630
Yui N, Dijkstra PJ, Feijen J (1990) Stereo block copolymers of l- and d-lactides. Macromol Chem 191:481–488
Li L, Zhong Z, Jeu WH, Dijkstra PJ, Feijen J (2004) Crystal structure and morphology of poly(l-lactide-b-d-lactide) diblock copolymers. Macromolecules 37:8641–8646
Hu J, Tang Z, Qiu X, Pang X, Yang Y, Chen X, Jing X (2005) Formation of flower- or cake-shaped stereocomplex particles from the stereo multiblock copoly(rac-lactide)s. Biomacromolecules 6:2843–2850
Hirata M, Kobayashi K, Kimura Y (2010) Synthesis and properties of high-molecular-weight stereo di-block polylactides with nonequivalent D/L ratios. J Polym Sci, Part A: Polym Chem 48:794–801
Othma, N, Xu C, Mehrkhodavandi P. Hatzikiriakos SG (2012) Thermorheological and mechanical behavior of polylactide and its enantiomeric diblock copolymers and blends. Polymer 53:2443–2452
Aluthge DC, Xu C, Othman N, Noroozi N, Hatzikiriakos SG, Mehrkhodavandi P (2013) PLA–PHB–PLA Triblock copolymers: synthesis by sequential addition and investigation of mechanical and rheological properties. Macromolecules 46:3965–3974
Tsuji H, Tajima T (2014) Crystallization behavior of stereo diblock poly(lactide)s with relatively short poly(d-lactide) segment from partially melted state. Macromol Mater Eng 299:1089–1105
Mincheva R, Leclére Ph, Habibi Y, Raquez JM, Dubois P (2014) Preparation of narrowly dispersed stereocomplex nanocrystals: a step towards all-poly(lactic acid) nanocomposites. J Mater Chem A 2:7402–7409
Biela T, Duda A, Penczek S (2006) Enhanced melt stability of star-shaped stereocomplexes as compared with linear stereocomplexes. Macromolecules 39:3710–3713
Isono T, Kondo Y, Otsuka I, Nishiyama Y, Borsali R, Kakuchi T, Satoh T (2013) Synthesis and stereocomplex formation of star-shaped stereoblock polylactides consisting of poly(l-lactide) and poly(d-lactide) arms. Macromolecules 46:8509–8518
Shao J, Tang Z, Sun J, Li G, Chen X (2014) Linear and 4-armed poly(l-lactide)-block-poly(d-lactide) copolymers and their stereocomplexation with poly(lactide). J Polym Sci, Part B: Polym Phys 52:1560–1567
Tsuji H, Yamashita Y (2014) Highly accelerated stereocomplex crystallization by blending star-shaped 4-armed stereo diblock poly(lactide)s with poly(d-lactide) and poly(l-lactide) cores. Polymer 55:6444–6450
Ma Y, Li W, Li L, Fan Z, Li S (2014) Stereocomplexed three-arm PPO–PDLA–PLLA copolymers: synthesis via an end-functionalized initiator. Eur Polym J 55:27–34
Isono T, Kondo Y, Ozawa S, Chen Y, Sakai R, Sato S, Tajima K, Kakuchi T, Satoh T (2014) Stereoblock-like brush copolymers consisting of poly(l-lactide) and poly(d-lactide) side chains along poly(norbornene) backbone: synthesis, stereocomplex formation, and structure–property relationship. Macromolecules 47:7118–7128
Sugai N, Yamamoto T, Tezuka Y (2012) Synthesis of orientationally isomeric cyclic stereoblock polylactides with head-to-head and head-to-tail linkages of the enantiomeric segments. ACS Macro Lett 1:902–906
Shibata M, Katoh M, Takase H, Shibita A (2015) Stereocomplex formation in stereoblock copolymer networks composed of 4-armed star-shaped lactide oligomers and a 2-armed ɛ-caprolactone oligomer. Polym Chem 6:4123–4132
Shibita A, Kawasaki S, Shimasaki T, Teramoto N, Shibata M (2016) Stereocomplexation in copolymer networks incorporating enantiomeric glycerol-based 3-armed lactide oligomers and a 2-armed ɛ-caprolactone oligomer. Materials 9:591. doi:10.3390/ma9070591
Shibita A, Shimasaki T, Teramoto N, Shibata M (2015) Conetworks composed of 4-armed star-shaped l-lactide oligomer and 4-armed star-shaped ɛ-caprolactone oligomer. Polymer 74:54–62
Li Y, Han C, Zhang X, Dong Q, Dong L (2013) Effects of molten poly(d, l-lactide) on nonisothermal crystallization in stereocomplex of poly(l-lactide) with poly(d-lactide). Thermochim Acta 573:193–199
Li Y, Han C, Bian XY, Dong Q, Zha H, Zhang X, Xu M, Dong L (2014) Miscibility, thermal properties and polymorphism of stereocomplexation of high-molecular-weight polylactide/poly(d, l-lactide) blends. Thermochim Acta 580:53–62
Tsuji H, Nakano M, Hashimoto M, Takashima K, Katsura S, Mizuno A (2006) Electrospinning of poly(lactic acid) stereocomplex nanofibers. Biomacromolecules 7:3316–3320
Petchsuk A, Buchatip S, Supmak W, Opaprakasit M, Opaprakasit P (2014) Preparation and properties of multi-branched poly(d-lactide) derived from polyglycidol and its stereocomplex blends. Express Polym Lett 8:779–789
Fukushima K, Kimura Y (2006) Stereocomplexed polylactides (Neo-PLA) as high-performance bio-based polymers: their formation, properties, and application. Polym Int 55:626–642
Hoogsteen W, Postema AR, Pennings AJ, Brinke GT, Zugenmaier P (1990) Crystal structure, conformation and morphology of solution-spun poly(l-lactide) fibers. Macromolecules 23:634–642
Chen CC, Chueh JY, Tseng H, Huang HM, Lee SY (2003) Preparation and characterization of biodegradable PLA polymeric blends. Bimater 24:1167–1173
Kopinke FD, Remmler M, Mackenzie K, Moder M, Wachsen O (1996) Thermal decomposition of biodegradable polyesters-II. Poly(lactic acid). Polym Degrad Stab 53:329–342
Wachsen O, K. Reichert H, Kruger RP, Much H, Schulz G (1997) Thermal decomposition of biodegradable polyesters-III. Studies on the mechanisms of thermal degradation of oligo-l-lactide using SEC, LACCC and MALDI-TOF-MS. Polym Degrad Stab 55:225–231
Acknowledgements
We thank Dr. Naozumi Teramoto and Dr. Toshiaki Shimasaki of our department for their helpful suggestions. We are also grateful to Mr. Ryusuke Osada of the Material Analysis Center at the Chiba Institute of Technology for assisting in the XRD analysis reported here.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shibita, A., Mizumura, Y. & Shibata, M. Stereocomplex crystallization behavior and physical properties of polyesterurethane networks incorporating diglycerol-based enantiomeric 4-armed lactide oligomers and a 1,3-propanediol-based 2-armed rac-lactide oligomer. Polym. Bull. 74, 3139–3160 (2017). https://doi.org/10.1007/s00289-016-1890-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-016-1890-1