Abstract
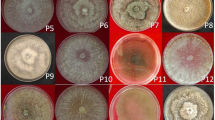
Penicillium is one of the most important postharvest pathogens of citrus fruits worldwide. It induces blue or green mold disease, a decay that can lead to significant economic losses during storage. Based on internal transcribed spacer (ITS) sequences, seven Penicillium species and one closely related Talaromyces variabilis were identified from 30 rotten samples of citrus fruits marketed in Qena. Penicillium expansum was the most common species, recovered from 16.7% of the samples, followed by P. chrysogenum (10%) and P. polonicum (10%). Sixteen isolates were tested through inoculation on healthy citrus fruits; the data exhibited that 68.7% of isolates were highly virulent. A “Specific Gene Random Primer Polymerase Chain Reaction (SGRP-PCR)” marker technique indicated that the genetic similarity among P. expasum ranged from 49.4 to 85.7%, and a relatively correlation was found between SGRP band profile and species origin. Patulin was detected in 40% of P. expansum isolates. This study provided a useful molecular approach to identify different Penicillium species by sequencing ITS region, focus on the pathogenicity, compare between P. expansum isolates and their ability in patulin production.




Similar content being viewed by others
References
Terol J, Conesa A, Colmenero JM, Cercos M, Tadeo F, Agusti J et al (2007) Analysis of 13000 unique citrus clusters associated with fruit quality, production and salinity tolerance. BMC Genomics 8:31
Dillard CJ (2000) Phytochemicals: nutraceuticals and human health. J Sci Food Agric 80:1744–1756
Jurick IIWM, Yu J, Bennett JW (2016) Blue mould to genomics and beyond: insights into the biology and virulence of phytopathogenic Penicillium species. In: de Vries RP, Gelber IB, Anderson MR (eds) Aspergillus and Penicillium in the Post-Genmic Era. Caister Academic Press, Norfolk, UK, p 210
Barkai-Golan R, Paster N (2008) Mouldy fruits and vegetables as a source of mycotoxins: part 1. World Mycotoxin J 1:147–159
Refai M, El-Yazid HA, Tawakko W (2015) Book Monograph on the genus Penicillium. A guide for historical, classification and identification of penicilli, their industrial applications and detrimental effects. Department of Microbiology, Faculty of Veterinary Medicine, Cairo University, pp. 1–157.
Visagie C, Houbraken J, Frisvad JC, Hong SB, Klaassen C, Perrone G et al (2014) Identification and nomenclature of the genus Penicillium. Stud Mycol 78:343–371
Raper KB, Thom C (1949) Amanual of the Penicillia. Williams and Wilkins Co, Baltimore, Maryland
Pitt JI (2000) A laboratory guide to common Penicillium species. 1988; North Ryde, N.S.W., Australia: commonwealth scientific and industrial research organization, Division of Food Processing.
Samson R, Ribera F, Sanchis V, Canela R (1995) Introduction to food-borne fungi. Centraalbureau voor Schimmelcultures, Baarn, Wageningen
Pitt JI, Hocking AD (1997) Fungi and food spoilage. Blackie Academic and Professional New South Wales, Australia
Pianzzola MJ, Moscatelli M, Vero S (2004) Characterization of Penicillium isolates associated with blue mold on apple in Uruguay. Plant Dis 88:23–28
Akhtar N, Anjum T, Jabeen R (2013) Isolation and identification of storage fungi from citrus sampled from major growing areas of Punjab, Pakistan. Int J Agric Biol 15(6):1283–1288
Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA et al (2012) Fungal barcoding consortium. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. PNAS 109:6241–6246
Abdel-Azeem AM, Gherbawy YA, Sabry AM (2016) Enzyme profiles and genotyping of Chaetomium globosum isolates from various substrates. Plant Biosyst Int J Plant Biol 150:420–428
Yin G, Zhang Y, Pennerman KK, Wu G, Hua SST, Yu J et al (2017) Characterization of blue mold Penicillium species isolated from stored fruits using multiple highly conserved loci. J Fungi (Basel) 3:12
Baiyewu RA, Amusa NA, Ayoola OA, Babalola OO (2007) Survey of the post-harvest diseases and aflatoxin contamination of marketed pawpaw fruit (Carica papaya L) in South Western Nigeria. Afr J Agric Res 2(4):178–181
Samson RA, Varga J (2007) (Eds.). Aspergillus systematics in the genomic era (No. 59). Utrecht: CBS Fungal Biodiversity Centre.
Day JP, Shattock RC (1997) Aggressiveness and other factors relating to displacement of populations of Phytophthora infestans in England and Wales. Eur J Plant Pathol 103:379–391
White TJ, Bruns T, Lee SJ, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocol 18:315–322
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Lui LH, Kushalappa AC (2002) Response surface models to predict potato tuber infection by Fusarium sambucinum from duration of wetness and temperature, and dry rot lesion expansion from storage time and temperature. Int J Food Microbiol 76:19–25
Nash SM, Snyder WC (1962) Quantitative estimations by plate counts of propagules of the bean root rot Fusarium in field soils. Phytopathology 52:567–572
Yli-Mattila T, Ward TJ, O’Donnell K, Proctor RH, Burkin AA, Kononenko GP et al (2011) Fusarium sibiricum sp. nov, a novel type A trichothecene-producing Fusarium from northern Asia closely related to F. sporotrichioides and F. langsethiae. Int J Food Microbiol 147:58–68
Peterson SW, Vega FE, Posada F (2005) Penicillium coffeae, a new endophytic species isolated from a coffee plant and its phylogenetic relationship to P. fellutanum, P. thiersii and P. brocae based on parsimony analysis of multilocus DNA sequences. Mycologia 97:659–666
Rehner SA, Buckley E (2005) A beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97:84–98
Fredj SMB, Chebil S, Mliki A (2009) Isolation and characterization of ochratoxin A and aflatoxin B1 producing fungi infecting grapevines cultivated in Tunisia. Afr J Microbiol Res 3:523–527
Larous L, Hendel N, Abood JK, Ghoul M (2007) The growth and production of patulin mycotoxin by Penecillium expansum on apple fruits and its control by the use of propionic acid and sodium benzoate. Arab J Pl Prot 25:123–128
Filtenborg O, Frisvad JC, Thrane U (1996) Moulds in food spoilage. Int J Food Microbiol 33:85–102
Bukar A, Mukhtar MD, Adamu S (2009) Isolation and identification of postharvest spoilage fungi associated with sweet oranges (Citrus sinensis) traded in Kano metropolis. BAJOPAS 2:122–124
Vico I, Duduk N, Vasić M, Nikolić M (2014) Identification of Penicillium expansum causing postharvest blue mold decay of apple fruit. Pesticidii fitomedicina 29:257–266
Houbraken J, Visagie CM, Meijer M, Frisvad JC, Busby PE, Pitt JI et al (2014) A taxonomic and phylogenetic revision of Penicillium section Aspergilloides. Stud Mycol 78:373–451
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118
Dupont J, Magnin S, Marti A (1999) Molecular tools for identification of Penicillium starter cultures used in the food industry. Int J Food Microbiol 49:109–118
Tiwari KL, Jadhav SK, Kumar A (2011) Morphological and molecular study of different Penicillium species. Middle-East J Sci Res 7:203–210
Gao X, Kolomiets MV (2009) Host-derived lipids and oxylipins are crucial signals in modulating mycotoxin production by fungi. Toxin Rev 28:79–88
Edel V (1998) Polymerase chain reaction in mycology — an overview. In: Bridge PD, Arora DK, Reddy CA, Elander RP (eds) Application of PCR in mycology. CAB International, Wallingford, pp 1–20
Doyle EM, Kelly CT, Fogarty WM (1989) The high maltose-producing α-amylase of Penicillium expansum. Appl Microbiol Biotechnol 3:492–496
Vidal A, Ouhibi S, Ghali R, Hedhili A, De Saeger S, De Boevre M (2019) The mycotoxin patulin: an updated short review on occurrence, toxicity and analytical challenges. Food Chem Toxicol 129:249–256
Saleh I, Goktepe I (2019) Health risk assessment of Patulin intake through apples and apple-based foods sold in Qatar. Heliyon 5:e02754
Acknowledgements
The authors are especially grateful and thankful to Taif University Researchers Supporting Project number (TURSP-2020/142), Taif University, Taif, Saudi Arabia for financial support and providing necessary facilities to carry out this research.
Author information
Authors and Affiliations
Contributions
All authors revised this manuscript. EGAEME and MAH participated in the methodology and designed the figures and tables of the manuscript. YAG planned the idea of manuscript and SH revised and supported the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. Research does not involve human participants and/or animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El-Dawy, E.G.A.E.M., Gherbawy, Y.A., Hassan, S. et al. Molecular Identification of Penicillium sp. Isolated from Citrus Fruits. Curr Microbiol 78, 1981–1990 (2021). https://doi.org/10.1007/s00284-021-02463-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-021-02463-3