Abstract
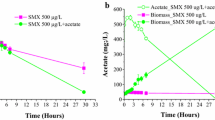
Two bacterial consortia capable of degrading SLES were isolated from a wastewater treatment plant. The two consortia consisted of three members, Acinetobacter calcoacetiacus and Klebsiella oxytoca in one co-culture (A-K) and Serratia odorifera in the second co-culture (S-A), which contains Acinetobacter calcoacetiacus as well. In all experiments, cells were grown on SLES (1000–7000 ppm) containing the M9 minimal medium as sole carbon source. The co-culture A-K demonstrated a higher growth rate (0.26 h−1) and significant greater viability than that of the co-culture S-A (0.21 h−1). Glucose, sucrose, maltose, mannitol, and succinic acid as carbon sources produced the same degradation rate (∼100 ppm/h) and enhanced the SLES degradation rate by 3-fold upon the control (without an added carbon source). In the case of the co-culture S-A, the situation was different; all the carbon sources being tested except maltose caused a repression in the degradation ability in a range between 25–100%. Maltose causes an enhancement by almost fivefold, compared with the positive control.




Similar content being viewed by others
Literature Cited
Swisher RD (1987) Surfactant biodegradation, 2nd ed. Marcel Dekker, New York, NY
Jerábková H, Králová B, Náhlik J (1999). Biofilm of Pseudomonas C12B on glass support as catalytic agent for continuous SDS removal. Int Biodeg Biodeter 44:233–241
Brandt KK, Hesselsøe MP, Roslev K, Henriksen, Sørensen J (2001) Toxic Effects of linear alkylbenzene sulfonate on metabolic activity, growth rate, and microcolony formation of Nitrosomonas and Nitrosospira Strains. Appl Environ Microbiol 67:2489–2498
Jiménez L, Breen A, Thomas N, Federle TW, Sayler GS. (1991) Mineralization of linear alkylbenzene sulfonate by a four-member aerobic bacterial consortium. Appl Environ Microbiol 57:1566–1569
Rapaport RA, Eckhoff WS (1990) Monitoring linear alkylbenzene sulfonate in the environment: 1973–1986. Environ Toxicol Chem 9:1245–1257
Karsa DR (1987) Industrial applications of surfactants. The proceedings of a symposium organized by the northwest region of the royal society of chemistry. London, England: The Royal of Chemistry, Burlinton House
Jakobi G, Löhr A (1987) Detergent ingredients. In: Gerhartz W (ed) Ullmannsencyclopedia of industrial chemistry, 5 ed. VCH, Weinheim, pp 338–372
Schleheck D, Lechner M, Sch?nenberger R, Suter MJF, Cook AM (2003) Desulfonation and degradation of sulfodiphenylethercarboxylates from linear alkyldiphenyletherdisulfonate surfactants. Appl Environ Microbiol 69:938–944
Al-Shamailah W (2005) Effect of wastewater treatment on the bacterial quality and quantity at Mutah University Plant. MSc Thesis, Mutah University, Jordan
Miller JH (1972). Experiment in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Khleifat K (2006) Biodegradation of linear alkylbenzene sulfonate by a two-member facultative anaerobic bacterial consortium. Enz Microbial Technol 39(5):1030–1035
Longwell J, Maniece WD (1955). Determination of anionic detergent in sewage effluents and river water. Analyst 80:167–171
Li Z, Rosen M (1981). Two-phase mixed indicator titration method for determination of anionic surfactants. Anal Chem J 53(9):1516–1519
Loh KC, Wang SJ (1998) Enhancement of biodegradation of phenol and a nongrowth substrate 4-chlorophenol by medium augmentation with conventional carbon sources. Biodegradation 8:329–338
Khosravi M, Ryan W, Webster DA, Stork BC (1990a). Variation of oxygen requirement with plasmid size in recombinant Escherichia coli. Plasmid 23:138–143
Fendinger NJ, Versteeg DJ, Weeg E, Dyer SD, Rapaport RA (1994). Environmental behaviour and fate of anionic surfactants. In Baker LA (ed) Environmental Chemistry of Lakes and Reservoirs, Advances in Chemistry Series No. 237. American Chemical Society, Washington, DC. pp 527–557
Nuhoglu A, Yalcin B (2004). Modeling of phenol removal in a batch reactor, Process Biochem 40:1233–1239
Zhang CL, Valsaraj KT, Constant WD, Roy D (1999). Aerobic biodegradation kinetics of four anionic and nonionic surfactants at sub- and supra-critical micelle concentrations. Water Res 33(1):115–124
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khleifat, K.M. Biodegradation of Sodium Lauryl Ether Sulfate (SLES) by Two Different Bacterial Consortia. Curr Microbiol 53, 444–448 (2006). https://doi.org/10.1007/s00284-006-0266-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-006-0266-4