Abstract
Purpose
Docetaxel is used to treat many cancers, and neutropenia is the dose-limiting factor for its clinical use. A population pharmacokinetic–pharmacodynamic (PK–PD) model was introduced to predict the development of docetaxel-induced neutropenia in Japanese patients with non-small cell lung cancer (NSCLC).
Methods
Forty-seven advanced or recurrent Japanese patients with NSCLC were enrolled. Patients received 50 or 60 mg/m2 docetaxel as monotherapy, and blood samples for a PK analysis were collected up to 24 h after its infusion. Laboratory tests including absolute neutrophil count data and demographic information were used in population PK–PD modeling. The model was built by NONMEM 7.2 with a first-order conditional estimation using an interaction method. Based on the final model, a Monte Carlo simulation was performed to assess the impact of covariates on and the predictability of neutropenia.
Results
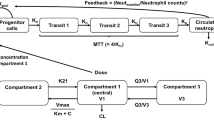
A three-compartment model was employed to describe PK data, and the PK model adequately described the docetaxel concentrations observed. Serum albumin (ALB) was detected as a covariate of clearance (CL): CL (L/h) = 32.5 × (ALB/3.6)0.965 × (WGHT/70)3/4. In population PK–PD modeling, a modified semi-mechanistic myelosuppression model was applied, and characterization of the time course of neutrophil counts was adequate. The covariate selection indicated that α1-acid glycoprotein (AAG) was a predictor of neutropenia. The model-based simulation also showed that ALB and AAG negatively correlated with the development of neutropenia and that the time course of neutrophil counts was predictable.
Conclusion
The developed model may facilitate the prediction and care of docetaxel-induced neutropenia.




Similar content being viewed by others
References
Ringel I, Horwitz SB (1991) Studies with RP 56976 (taxotere): a semisynthetic analogue of taxol. J Natl Cancer Inst 83(4):288–291
Nieuweboer AJ, de Morrée ES, de Graan A-JMJ, Sparreboom A, de Wit R, Mathijssen RH (2015) Inter-patient variability in docetaxel pharmacokinetics: a review. Cancer Treat Rev 41(7):605–613
Kenmotsu H, Tanigawara Y (2015) Pharmacokinetics, dynamics and toxicity of docetaxel: why the Japanese dose differs from the Western dose. Cancer Sci 106(5):497–504
Crawford J, Dale DC, Lyman GH (2004) Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer 100(2):228–237
Fontanella C, Bolzonello S, Lederer B, Aprile G (2014) Management of breast cancer patients with chemotherapy-induced neutropenia or febrile neutropenia. Breast Care 9(4):239–245
Bruno R, Hille D, Riva A, Vivier N, ten Bokkel Huinnink WW, van Oosterom AT, Kaye SB, Verweij J, Fossella FV, Valero V, Rigas JR, Seidman AD, Chevallier B, Fumoleau P, Burris HA, Ravdin PM, Sheiner LB (1998) Population pharmacokinetics/pharmacodynamics of docetaxel in phase II studies in patients with cancer. J Clin Oncol 16(1):187–196
Baker SD, Verweij J, Cusatis GA, van Schaik RH, Marsh S, Orwick SJ, Franke RM, Hu S, Schuetz EG, Lamba V, Messersmith WA, Wolff AC, Carducci MA, Sparreboom A (2009) Pharmacogenetic pathway analysis of docetaxel elimination. Clin Pharmacol Ther 85(2):155–163
Franke RM, Carducci MA, Rudek MA, Baker SD, Sparreboom A (2010) Castration-dependent pharmacokinetics of docetaxel in patients with prostate cancer. J Clin Oncol 28(30):4562–4567
Fajac A, Gligorov J, Rezai K, Lévy P, Lévy E, Selle F, Beerblock K, Avenin D, Saintigny P, Hugonin S, Bernaudin J-FF, Lokiec F (2010) Effect of ABCB1 C3435T polymorphism on docetaxel pharmacokinetics according to menopausal status in breast cancer patients. Br J Cancer 103(4):560–566
Goh B-CC, Lee S-CC, Wang L-ZZ, Fan L, Guo J-YY, Lamba J, Schuetz E, Lim R, Lim H-LL, Ong A-BB, Lee H-SS (2002) Explaining interindividual variability of docetaxel pharmacokinetics and pharmacodynamics in Asians through phenotyping and genotyping strategies. J Clin Oncol 20(17):3683–3690
Slaviero KA, Clarke SJ, McLachlan AJ, Blair EY, Rivory LP (2004) Population pharmacokinetics of weekly docetaxel in patients with advanced cancer. Br J Clin Pharmacol 57(1):44–53
Minami H, Kawada K, Sasaki Y, Tahara M, Igarashi T, Itoh K, Fujii H, Saeki T, Ozawa K, Sato H (2009) Population pharmacokinetics of docetaxel in patients with hepatic dysfunction treated in an oncology practice. Cancer Sci 100(1):144–149
Baker SD, Li J, ten Tije AJ, Figg WD, Graveland W, Verweij J, Sparreboom A (2005) Relationship of systemic exposure to unbound docetaxel and neutropenia. Clin Pharmacol Ther 77(1):43–53
Urien S, Barré J, Morin C, Paccaly A, Montay G, Tillement JP (1996) Docetaxel serum protein binding with high affinity to alpha 1-acid glycoprotein. Invest New Drugs 14(2):147–151
De Graan A-JMJ, Lancaster CS, Obaidat A, Hagenbuch B, Elens L, Friberg LE, de Bruijn P, Hu S, Gibson AA, Bruun GH, Corydon TJ, Mikkelsen TS, Walker AL, Du G, Loos WJ, van Schaik RH, Baker SD, Mathijssen RH, Sparreboom A (2012) Influence of polymorphic OATP1B-type carriers on the disposition of docetaxel. Clin Cancer Res 18(16):4433–4440
Iusuf D, Hendrikx JJ, van Esch A, van de Steeg E, Wagenaar E, Rosing H, Beijnen JH, Schinkel AH (2015) Human OATP1B1, OATP1B3 and OATP1A2 can mediate the in vivo uptake and clearance of docetaxel. Int J Cancer 136(1):225–233
Shou M, Martinet M, Korzekwa KR, Krausz KW, Gonzalez FJ, Gelboin HV (1998) Role of human cytochrome P450 3A4 and 3A5 in the metabolism of taxotere and its derivatives: enzyme specificity, interindividual distribution and metabolic contribution in human liver. Pharmacogenetics 8(5):391–401
Wils P, Phung-Ba V, Warnery A, Lechardeur D, Raeissi S, Hidalgo IJ, Scherman D (1994) Polarized transport of docetaxel and vinblastine mediated by P-glycoprotein in human intestinal epithelial cell monolayers. Biochem Pharmacol 48(7):1528–1530
Huisman MT, Chhatta AA, van Tellingen O, Beijnen JH, Schinkel AH (2005) MRP2 (ABCC2) transports taxanes and confers paclitaxel resistance and both processes are stimulated by probenecid. Int J Cancer 116(5):824–829
Frederiks CN, Lam SW, Guchelaar HJ, Boven E (2015) Genetic polymorphisms and paclitaxel- or docetaxel-induced toxicities: a systematic review. Cancer Treat Rev 41(10):935–950
Kiyotani K, Mushiroda T, Kubo M, Zembutsu H, Sugiyama Y, Nakamura Y (2008) Association of genetic polymorphisms in SLCO1B3 and ABCC2 with docetaxel-induced leukopenia. Cancer Sci 99(5):967–972
Engels FK, Mathot RAA, Loos WJ, van Schaik RH, Verweij J (2006) Influence of high-dose ketoconazole on the pharmacokinetics of docetaxel. Cancer Biol Ther 5(7):833–839
Deeken JF, Cormier T, Price DK, Sissung TM, Steinberg SM, Tran K, Liewehr DJ, Dahut WL, Miao X, Figg WD (2010) A pharmacogenetic study of docetaxel and thalidomide in patients with castration-resistant prostate cancer using the DMET genotyping platform. Pharmacogenomics J 10(3):191–199
Yano R, Konno A, Watanabe K, Tsukamoto H, Kayano Y, Ohnaka H, Goto N, Nakamura T, Masada M (2013) Pharmacoethnicity of docetaxel-induced severe neutropenia: integrated analysis of published phase II and III trials. Int J Clin Oncol 18(1):96–104
Puisset F, Alexandre J, Treluyer J-MM, Raoul V, Roché H, Goldwasser F, Chatelut E (2007) Clinical pharmacodynamic factors in docetaxel toxicity. Br J Cancer 97(3):290–296
Sheiner LB, Ludden TM (1992) Population pharmacokinetics/dynamics. Annu Rev Pharmacol Toxicol 32:185–209
Jonsson EN, Karlsson MO (1999) Xpose—an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed 58(1):51–64
Maruyama R, Nishiwaki Y, Tamura T, Yamamoto N, Tsuboi M, Nakagawa K, Shinkai T, Negoro S, Imamura F, Eguchi K, Takeda K, Inoue A, Tomii K, Harada M, Masuda N, Jiang H, Itoh Y, Ichinose Y, Saijo N, Fukuoka M (2008) Phase III study, V-15-32, of gefitinib versus docetaxel in previously treated Japanese patients with non-small-cell lung cancer. J Clin Oncol 26(26):4244–4252
Karlsson MO, Savic RM (2007) Diagnosing model diagnostics. Clin Pharmacol Ther 82(1):17–20
Hooker AC, Staatz CE, Karlsson MO (2007) Conditional weighted residuals (CWRES): a model diagnostic for the FOCE method. Pharm Res 24(12):2187–2197
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO (2011) Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J 13(2):143–151
Lindbom L, Pihlgren P, Jonsson EN, Jonsson N (2005) PsN-Toolkit—a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed 79(3):241–257
Lindbom L, Ribbing J, Jonsson EN (2004) Perl-speaks-NONMEM (PsN)—a Perl module for NONMEM related programming. Comput Methods Programs Biomed 75(2):85–94
Friberg LE, Henningsson A, Maas H, Nguyen L, Karlsson MO (2002) Model of chemotherapy-induced myelosuppression with parameter consistency across drugs. J Clin Oncol 20(24):4713–4721
Yamamoto N, Tamura T, Kamiya Y, Sekine I, Kunitoh H, Saijo N (2000) Correlation between docetaxel clearance and estimated cytochrome P450 activity by urinary metabolite of exogenous cortisol. J Clin Oncol 18(11):2301–2308
Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O’Rourke M, Levitan N, Gressot L, Vincent M, Burkes R, Coughlin S, Kim Y, Berille J (2000) Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 18(10):2095–2103
Krzakowski M, Ramlau R, Jassem J, Szczesna A, Zatloukal P, Von Pawel J, Sun X, Bennouna J, Santoro A, Biesma B, Delgado FMM, Salhi Y, Vaissiere N, Hansen O, Tan E-HH, Quoix E, Garrido P, Douillard J-YY (2010) Phase III trial comparing vinflunine with docetaxel in second-line advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy. J Clin Oncol 28(13):2167–2173
Kawaguchi T, Ando M, Asami K, Okano Y, Fukuda M, Nakagawa H, Ibata H, Kozuki T, Endo T, Tamura A, Kamimura M, Sakamoto K, Yoshimi M, Soejima Y, Tomizawa Y, Isa S, Takada M, Saka H, Kubo A (2014) Randomized phase III trial of erlotinib versus docetaxel as second- or third-line therapy in patients with advanced non-small-cell lung cancer: docetaxel and Erlotinib Lung Cancer Trial (DELTA). J Clin Oncol 32(18):1902–1908
Ozawa K, Minami H, Sato H (2007) Population pharmacokinetic and pharmacodynamic analysis for time courses of docetaxel-induced neutropenia in Japanese cancer patients. Cancer Sci 98(12):1985–1992
Quartino AL, Friberg LE, Karlsson MO (2012) A simultaneous analysis of the time-course of leukocytes and neutrophils following docetaxel administration using a semi-mechanistic myelosuppression model. Invest New Drugs 30(2):833–845
Quartino AL, Karlsson MO, Lindman H, Friberg LE (2014) Characterization of endogenous G-CSF and the inverse correlation to chemotherapy-induced neutropenia in patients with breast cancer using population modeling. Pharm Res 31(12):3390–3403
Hansson EK, Wallin JE, Lindman H, Sandström M, Karlsson MO, Friberg LE (2010) Limited inter-occasion variability in relation to inter-individual variability in chemotherapy-induced myelosuppression. Cancer Chemother Pharmacol 65(5):839–848
Hansson EK, Friberg LE (2012) The shape of the myelosuppression time profile is related to the probability of developing neutropenic fever in patients with docetaxel-induced grade IV neutropenia. Cancer Chemother Pharmacol 69(4):881–890
Kuderer NM, Dale DC, Crawford J, Lyman GH (2007) Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortalityin adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol 25(21):3158–3167
Cullen M, Baijal S (2009) Prevention of febrile neutropenia: use of prophylactic antibiotics. Br J Cancer 101:S11–S14
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional and/or National Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fukae, M., Shiraishi, Y., Hirota, T. et al. Population pharmacokinetic–pharmacodynamic modeling and model-based prediction of docetaxel-induced neutropenia in Japanese patients with non-small cell lung cancer. Cancer Chemother Pharmacol 78, 1013–1023 (2016). https://doi.org/10.1007/s00280-016-3157-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-3157-9