Abstract
Purpose
The aims of this dose-escalating phase I study were to determine the maximum tolerable dose (MTD) and recommended dose (RD) of 5-fluorouracil (5-FU), docetaxel, and nedaplatin (UDON) combination therapy for future phase II studies, and to evaluate the safety and efficacy of this regimen in patients with untreated recurrent or metastatic esophageal cancer.
Methods
Patients were administered 5-FU on days 1–5, docetaxel on days 1 and 15, and nedaplatin on day 1 at 4-week intervals. The dose levels of 5-FU/docetaxel/nedaplatin were escalated as follows (mg/m2): level 1, 800/30/80; level 2, 800/30/90; and level 3, 800/35/90. Toxicity was evaluated using Common Terminology Criteria for Adverse Events version 4.0.
Results
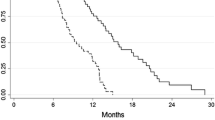
Overall, nine patients were enrolled in this study. All patients had an Eastern Cooperative Oncology Group performance status of 0 or 1 and were diagnosed with squamous cell carcinoma. No dose-limiting toxicity was observed at any level, and planned dose escalation was completed without reaching the MTD. No grade 4 or higher toxicity was observed in this study. The observed grade 3 hematological toxicities included neutropenia in five patients (55.6 %) and leukopenia in three patients (33.3 %). None of the patients developed febrile neutropenia, and no grade 3 or 4 non-hematological toxicities were observed. The overall response rate was 77.8 %, including two complete responses, and the disease control rate was 100 %.
Conclusion
The RD of UDON was identified as level 3. The good tolerability and high antitumor efficacy of this regimen warrant further evaluation in this setting.



Similar content being viewed by others
References
Kamangar F, Dores GM, Anderson WF (2006) Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 24:2137–2150
Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA Jr, Al-Sarraf M, Byhardt R, Russell AH, Beitler JJ, Spencer S, Asbell SO, Graham MV, Leichman LL (1999) Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiat Ther Oncol Group. JAMA 281:1623–1627
Iizuka T, Kakegawa T, Ide H, Ando N, Watanabe H, Tanaka O, Takagi I, Isono K, Ishida K, Arimori M et al (1992) Phase II evaluation of cisplatin and 5-fluorouracil in advanced squamous cell carcinoma of the esophagus: a Japanese Esophageal Oncology Group Trial. Jpn J Clin Oncol 22:172–176
Anand BS, Saeed ZA, Michaletz PA, Winchester CB, Doherty MA, Liem JH, Graham DY (1998) A randomized comparison of dilatation alone versus dilatation plus laser in patients receiving chemotherapy and external beam radiation for esophageal carcinoma. Dig Dis Sci 43:2255–2260
Riccardi D, Allen K (1999) Nutritional management of patients with esophageal and esophagogastric junction cancer. Cancer Control 6:64–72
Marin FA, Lamonica-Garcia VC, Henry MA, Burini RC (2010) Grade of esophageal cancer and nutritional status impact on postsurgery outcomes. Arq Gastroenterol 47:348–353
Osaka Y, Shinohara M, Hoshino S, Ogata T, Takagi Y, Tsuchida A, Aoki T (2011) Phase II study of combined chemotherapy with docetaxel, CDDP and 5-FU for highly advanced esophageal cancer. Anticancer Res 31:633–638
Hironaka S, Tsubosa Y, Mizusawa J, Kii T, Kato K, Tsushima T, Chin K, Tomori A, Okuno T, Taniki T, Ura T, Matsushita H, Kojima T, Doki Y, Kusaba H, Fujitani K, Taira K, Seki S, Nakamura T, Kitagawa Y, Japan Esophageal Oncology Group/Japan Clinical Oncology G (2014) Phase I/II trial of 2-weekly docetaxel combined with cisplatin plus fluorouracil in metastatic esophageal cancer (JCOG0807). Cancer Sci 105:1189–1195
Yokota T, Hatooka S, Ura T, Abe T, Takahari D, Shitara K, Nomura M, Kondo C, Mizota A, Yatabe Y, Shinoda M, Muro K (2011) Docetaxel plus 5-fluorouracil and cisplatin (DCF) induction chemotherapy for locally advanced borderline-resectable T4 esophageal cancer. Anticancer Res 31:3535–3541
Yamasaki M, Miyata H, Tanaka K, Shiraishi O, Motoori M, Peng YF, Yasuda T, Yano M, Shiozaki H, Mori M, Doki Y (2011) Multicenter phase I/II study of docetaxel, cisplatin and fluorouracil combination chemotherapy in patients with advanced or recurrent squamous cell carcinoma of the esophagus. Oncology 80:307–313
Sasaki Y, Tamura T, Eguchi K, Shinkai T, Fujiwara Y, Fukuda M, Ohe Y, Bungo M, Horichi N, Niimi S et al (1989) Pharmacokinetics of (glycolate-0,0′)-diammine platinum (II), a new platinum derivative, in comparison with cisplatin and carboplatin. Cancer Chemother Pharmacol 23:243–246
Suzumura Y, Kato T, Ueda R, Ota K (1989) Effect of treatment schedule on antitumor activity of glycolate-0,0′-diammineplatinum(II), a new platinum derivative: comparison with cis-diamminedichloroplatinum(II). Anticancer Res 9:1083–1088
Sasaki Y, Fukuda M, Morita M, Shinkai T, Eguchi K, Tamura T, Ohe Y, Yamada K, Kojima A, Nakagawa K et al (1990) Prediction from creatinine clearance of thrombocytopenia and recommended dose in patients receiving (glycolato-O, O’)-diammine platinum (II) (NSC 375101D). Jpn J Cancer Res 81:196–200
Fukuda M, Shinkai T, Eguchi K, Sasaki Y, Tamura T, Ohe Y, Kojima A, Oshita F, Hara K, Saijo N (1990) Phase II study of (glycolate-O, O′) diammineplatinum(II), a novel platinum complex, in the treatment of non-small-cell lung cancer. Cancer Chemother Pharmacol 26:393–396
Akaza H, Togashi M, Nishio Y, Miki T, Kotake T, Matsumura Y, Yoshida O, Aso Y (1992) Phase II study of cis-diammine(glycolato)platinum, 254-S, in patients with advanced germ-cell testicular cancer, prostatic cancer, and transitional-cell carcinoma of the urinary tract. 254-S Urological Cancer Study Group. Cancer Chemother Pharmacol 31:187–192
Taguchi T, Wakui A, Nabeya K, Kurihara M, Isono K, Kakegawa T, Ota K (1992) A phase II clinical study of cis-diammine glycolato platinum, 254-S, for gastrointestinal cancers. 254-S Gastrointestinal Cancer Study Group. Gan To Kagaku Ryoho 19:483–488
Yoshioka T, Gamoh M, Shineha R, Ishibashi S, Shibata H, Suzuki T, Murakawa Y, Kato S, Shimodaira H, Kato S, Ishioka C, Kanamaru R (1999) A new combination chemotherapy with cis-diammine-glycolatoplatinum (Nedaplatin) and 5-fluorouracil for advanced esophageal cancers. Intern Med 38:844–848
Kato K, Muro K, Ando N, Nishimaki T, Ohtsu A, Aogi K, Aoyama N, Nagai K, Kato H (2014) A phase II study of nedaplatin and 5-fluorouracil in metastatic squamous cell carcinoma of the esophagus: The Japan Clinical Oncology Group (JCOG) Trial (JCOG 9905-DI). Esophagus 11(3):183–188
Muro K, Hamaguchi T, Ohtsu A, Boku N, Chin K, Hyodo I, Fujita H, Takiyama W, Ohtsu T (2004) A phase II study of single-agent docetaxel in patients with metastatic esophageal cancer. Ann Oncol 15:955–959
Ajani JA (2002) Docetaxel for gastric and esophageal carcinomas. Oncology (Williston Park) 16:89–96
Minamide J, Aoyama N, Takada K, Oota Y (2007) Evaluation of docetaxel, CDDP and 5-FU combined therapy as second-line chemotherapy for esophagus cancer. Gan To Kagaku Ryoho 34:49–52
Cen P, Ajani JA (2007) Medical treatment for advanced gastroesophageal adenocarcinoma. Curr Opin Gastroenterol 23:631–635
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Miyazaki T, Sohda M, Tanaka N, Suzuki S, Ieta K, Sakai M, Sano A, Yokobori T, Inose T, Nakajima M, Fukuchi M, Ojima H, Kato H, Kuwano H (2013) Phase I dose-escalation study of docetaxel, nedaplatin, and 5-fluorouracil combination chemotherapy in patients with advanced esophageal cancer. Cancer Chemother Pharmacol 71:853–857
Engels FK, Verweij J (2005) Docetaxel administration schedule: from fever to tears? A review of randomised studies. Eur J Cancer 41:1117–1126
Tebbutt NC, Cummins MM, Sourjina T, Strickland A, Van Hazel G, Ganju V, Gibbs D, Stockler M, Gebski V, Zalcberg J, Australasian Gastro-Intestinal Trials G (2010) Randomised, non-comparative phase II study of weekly docetaxel with cisplatin and 5-fluorouracil or with capecitabine in oesophagogastric cancer: the AGITG ATTAX trial. Br J Cancer 102:475–481
Overman MJ, Kazmi SM, Jhamb J, Lin E, Yao JC, Abbruzzese JL, Ho L, Ajani J, Phan A (2010) Weekly docetaxel, cisplatin, and 5-fluorouracil as initial therapy for patients with advanced gastric and esophageal cancer. Cancer 116:1446–1453
Acknowledgments
We thank Asami Kitano and Tomoka Yamada from the Department of Medical Oncology, Kinki University Faculty of Medicine, Osaka, Japan.
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ueda, S., Kawakami, H., Nishina, S. et al. Phase I trial of 5-FU, docetaxel, and nedaplatin (UDON) combination therapy for recurrent or metastatic esophageal cancer. Cancer Chemother Pharmacol 76, 279–285 (2015). https://doi.org/10.1007/s00280-015-2799-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-015-2799-3