Abstract
Background
Single-agent chemotherapy with third-generation non-platinum agents, such as docetaxel, vinorelbine, is a standard therapeutic option for elderly patients with non-small-cell lung cancer (NSCLC). Subset analysis of a previous phase III study comparing pemetrexed with docetaxel in the second-line setting showed the superiority of pemetrexed in an elderly (≥70) population in both efficacy and toxicity.
Patients and methods
This was a single-arm phase II study of pemetrexed in elderly (≥75) Japanese patients with advanced non-squamous NSCLC. Patients received four cycles of pemetrexed (500 mg/m2) every 3 weeks. The primary endpoint was the response rate, and secondary endpoints were safety and survival.
Results
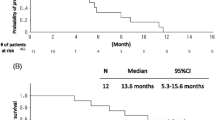
Twenty-eight patients were enrolled between January 2010 and April 2012. The median age of the patients was 77 years (range 75–88). All but one patient had adenocarcinoma histology. The median number of chemotherapy cycles administered was 4 (range, 1–12). Seventeen (60 %) patients completed four cycles of chemotherapy. Partial response was achieved in 7 patients (response rate: 25 %) and stable disease in 11 patients (disease control rate: 64 %). Median progression-free survival and overall survival were 3.3 and 17.5 months, respectively. Grade 3/4 neutropenia and thrombocytopenia were observed in 8 patients (29 %) and 2 (7 %), respectively. Non-hematologic toxicities were generally mild, and there were no treatment-related deaths.
Conclusions
Although this study did not meet our primary endpoint, pemetrexed showed favorable antitumor activity with mild toxicity in elderly patients with non-squamous NSCLC. Further investigations of pemetrexed in this population are warranted (UMIN-CTR number, 000002452).


Similar content being viewed by others
References
Gridelli C, Aapro M, Ardizzoni A, Balducci L, De Marinis F, Kelly K, Le Chevalier T, Manegold C, Perrone F, Rosell R, Shepherd F, De Petris L, Di Maio M, Langer C (2005) Treatment of advanced non-small-cell lung cancer in the elderly: results of an international expert panel. J Clin Oncol 23:3125–3137
Hutchins LF, Unger JM, Crowley JJ, Coltman CA Jr, Albain KS (1999) Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med 341:2061–2067
The Elderly Lung Cancer Vinorelbine Italian Study Group (1999) Effects of vinorelbine on quality of life and survival of elderly patients with advanced non-small-cell lung cancer. J Natl Cancer Inst 91:66–72
Gridelli C, Perrone F, Gallo C, Cigolari S, Rossi A, Piantedosi F, Barbera S, Ferraù F, Piazza E, Rosetti F, Clerici M, Bertetto O, Robbiati SF, Frontini L, Sacco C, Castiglione F, Favaretto A, Novello S, Migliorino MR, Gasparini G, Galetta D, Iaffaioli RV, Gebbia V, MILES Investigators (2003) Chemotherapy for elderly patients with advanced non-small-cell lung cancer: the Multicenter Italian Lung Cancer in the Elderly Study (MILES) phase III randomized trial. J Natl Cancer Inst 95:362–372
Kudoh S, Takeda K, Nakagawa K, Takada M, Katakami N, Matsui K, Shinkai T, Sawa T, Goto I, Semba H, Seto T, Ando M, Satoh T, Yoshimura N, Negoro S, Fukuoka M (2006) Phase III study of docetaxel compared with vinorelbine in elderly patients with advanced non-small-cell lung cancer: results of the West Japan Thoracic Oncology Group Trial (WJTOG 9904). J Clin Oncol 24:3657–3663
D’Addario G, Fruh M, Reck M, Baumann P, Klepetko W, Felip E, ESMO Guidelines Working Group (2010) Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 21(Suppl 5):v116–v119
de Marinis F, Rossi A, Di Maio M, Ricciardi S, Gridelli C (2011) Treatment of advanced non-small-cell lung cancer: Italian Association of Thoracic Oncology (AIOT) clinical practice guidelines. Lung Cancer 73:1–10
Muhsin M, Gricks C, Kirkpatrick P (2004) Pemetrexed disodium. Nat Rev Drug Discov 3:825–826
Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, Gatzemeier U, Tsao TC, Pless M, Muller T, Lim HL, Desch C, Szondy K, Gervais R, Shaharyar, Manegold C, Paul S, Paoletti P, Einhorn L, Bunn PA Jr (2004) Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 22:1589–1597
Weiss GJ, Langer C, Rosell R, Hanna N, Shepherd F, Einhorn LH, Nguyen B, Paul S, McAndrews P, Bunn PA Jr, Kelly K (2006) Elderly patients benefit from second-line cytotoxic chemotherapy: a subset analysis of a randomized phase III trial of pemetrexed compared with docetaxel in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol 24:4405–4511
Scagliotti G, Hanna N, Fossella F, Sugarman K, Blatter J, Peterson P, Simms L, Shepherd FA (2009) The differential efficacy of pemetrexed according to NSCLC histology: a review of two Phase III studies. Oncologist 14:253–263
Scagliotti G, Brodowicz T, Shepherd FA, Zielinski C, Vansteenkiste J, Manegold C, Simms L, Fossella F, Sugarman K, Belani CP (2011) Treatment-by-histology interaction analyses in three phase III trials show superiority of pemetrexed in nonsquamous non-small cell lung cancer. J Thorac Oncol 6:64–70
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Azzoli CG, Temin S, Aliff T, Baker S Jr, Brahmer J, Johnson DH, Laskin JL, Masters G, Milton D, Nordquist L, Pao W, Pfister DG, Piantadosi S, Schiller JH, Smith R, Smith TJ, Strawn JR, Trent D, Giaccone G, American Society of Clinical Oncology (2011) 2011 focused update of 2009 American Society of Clinical Oncology clinical practice guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol 29:3825–3831
Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH, Eastern Cooperative Oncology Group (2002) Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346:92–98
Ohe Y, Ohashi Y, Kubota K, Tamura T, Nakagawa K, Negoro S, Nishiwaki Y, Saijo N, Ariyoshi Y, Fukuoka M (2007) Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non-small-cell lung cancer: Four-Arm Cooperative Study in Japan. Ann Oncol 18:317–323
Pallis AG, Gridelli C, van Meerbeeck JP, Greillier L, Wedding U, Lacombe D, Welch J, Belani CP, Aapro M (2010) EORTC Elderly Task Force and Lung Cancer Group and International Society for Geriatric Oncology (SIOG) experts’ opinion for the treatment of non-small-cell lung cancer in an elderly population. Ann Oncol 21:692–706
Quoix E, Zalcman G, Oster JP, Westeel V, Pichon E, Lavolé A, Dauba J, Debieuvre D, Souquet PJ, Bigay-Game L, Dansin E, Poudenx M, Molinier O, Vaylet F, Moro-Sibilot D, Herman D, Bennouna J, Tredaniel J, Ducoloné A, Lebitasy MP, Baudrin L, Laporte S, Milleron B, Intergroupe Francophone de Cancérologie Thoracique (2011) Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet 378:1079–1088
Abe T, Yokoyama A, Takeda K, Ohe Y, Kudoh S, Ichinose Y, Okamoto H, Yamamoto N, Yoshioka H, Minato K, Sawa T, Iwamoto Y, Saka H, Mizusawa J, Shibata T, Nakamura S, Ando M, Nakagawa K, Saijo N, Tamura T (2011) Randomized phase III trial comparing weekly docetaxel (D)-cisplatin (P) combination with triweekly D alone in elderly patients (pts) with advanced non-small cell lung cancer (NSCLC): an intergroup trial of JCOG0803/WJOG4307L. J Clin Oncol 29:7509 (ASCO Meeting Abstracts)
Guetz G, Uzzan B, Nicolas P, Valeyre D, Sebbane G, Morere JF (2012) Comparison of the efficacy and safety of single-agent and doublet hemotherapy in advanced non-small cell lung cancer in the elderly: a meta-analysis. Crit Rev Oncol Hematol 84(3):340–349
Lilenbaum R, Zukin M, Pereira JR, Barrios CH, De Albuquerque Ribeiro R, Beato CAdM, Neron do Nascimento Y, Murad A, Franke FA, Precivale M, de Lima Araujo LH, Baldotto CSDR, Vieira FM, Small IA, Ferreira CGM (2012) A randomized phase III trial of single-agent pemetrexed (P) versus carboplatin and pemetrexed (CP) in patients with advanced non-small cell lung cancer (NSCLC) and performance status (PS) of 2. J Clin Oncol 30:7506 (ASCO Meeting Abstracts)
Dy GK, Molina JR, Qi Y, Ansari RH, Thomas SP, Ross HJ, Meyers JP, Adjei A, Mandrekar SJ, Adjei AA, North Central Cancer Treatment Group (2012) N0821: a phase II first-line study of a combination of pemetrexed (P), carboplatin (C), and bevacizumab (B) in elderly patients with good performance status (PS < 2). J Clin Oncol 30:7555 (ASCO Meeting Abstracts)
Kim YH, Yoh K, Niho S, Goto K, Ohmatsu H, Kubota K, Nishiwaki Y (2010) Trends in chemotherapy for elderly patients with advanced non-small-cell lung cancer. Respir Med 104:434–439
Paz-Ares L, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, Molinier O, Sahoo TP, Laack E, Reck M, Corral J, Melemed S, John W, Chouaki N, Zimmermann AH, Visseren-Grul C, Gridelli C (2012) Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol 13:247–255
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, Y.H., Hirabayashi, M., Kosaka, S. et al. Phase II study of pemetrexed as first-line treatment in elderly (≥75) non-squamous non-small-cell lung cancer: Kyoto Thoracic Oncology Research Group Trial 0901. Cancer Chemother Pharmacol 71, 1445–1451 (2013). https://doi.org/10.1007/s00280-013-2142-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2142-9