Abstract
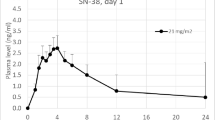
Patient’s preference is for oral chemotherapy when both oral and i.v. are available, provided that efficacy is equivalent. Reliable switch from oral to i.v. is possible if correspondence between respective doses has been established. Vinorelbine oral was developed as a line extension of VRL i.v. on the basis that similar AUCs result in similar activities. From a first crossover study on 24 patients receiving VRL 25 mg/m2 i.v. and 80 mg/m2 oral data extrapolation concluded on AUCs bioequivalence between Vinorelbine 30 mg/m2 i.v. and 80 mg/m2 oral. A new trial was performed to support this calculation. In a crossover design study on patients (PS 0-1) with advanced solid tumours (44% breast carcinoma), VRL was administered (30 mg/m2 i.v., 80 mg/m2 oral) with a standard meal and 5-HT3 antagonists, at 2 weeks interval. Pharmacokinetics was performed over 168 h and VRL was measured by LC-MS/MS. Statistics included bioequivalence tests. Forty-eight patients were evaluable for PK: median age 58 years (25–71), PS0/PS1: 20/28, M/F: 11/37. Mean AUCs were 1,230 ± 290 and 1,216 ± 521 ng/ml for i.v. and oral, respectively. The confidence interval of the AUC ratio (0.83–1.03) was within the required regulatory range (0.8–1.25) and proved the bioequivalence between the two doses. The absolute bioavailability was 37.8 ± 16.0%, and close to the value from the first study (40%). Patient tolerability was globally comparable between both forms with no significant difference on either haematological or non-haematological toxicities (grade 3–4). This new study, conducted on a larger population, confirmed the reliable dose correspondence previously established between vinorelbine 80 mg/m2 oral and 30 mg/m2 i.v.





Similar content being viewed by others
References
Bonneterre J, Chevalier B, Focan C, Mauriac L, Piccart M (2001) Phase I and pharmacokinetic study of weekly oral therapy with vinorelbine in patients with advanced breast cancer (ABC). Ann Oncol 12(12):1683–1691
Borner MM, Schoffski P, de Wit R, Caponigro F, Comella G, et al (2002) Patient preference and pharmacokinetics of oral modulated UFT versus intravenous fluorouracil and leucovorin: a randomised crossover trial in advanced colorectal cancer. Eur J Cancer 38(3):349–358
Bugat R, Variol P, Roché H, Fumoleau P, Robinet G, Sénac I (2002) The effect of food on pharmacokinetic profile of oral vinorelbine. Cancer Chemother Pharmacol 50:285–290
CPMP Working party on efficacy of medicinal products (Dec1991) Note for guidance: investigation of bioavailability and bioequivalence. III/54/89-EN
Crawford J, O’Rourke M, Schiller JH et al (1996) Randomized trial of vinorelbine compared with fluorouracil plus leucovorin in patients with stage IV NSCLC. J Clin Oncol 14(10):2774–2784
Depierre A, Chastang Cl, Quoix E, Lebeau B, Blanchon F, et al (1994) Vinorelbine versus vinorelbine plus cisplatin in advanced NSCLC: a randomized trial. Ann Oncol 5:37–42
Diletti E, Hauschke D, Steinnijans VW (1991) Sample size determination for bioequivalence assessment by mean of confidence intervals. Int J Clin Pharmacol Ther 30(1):S51–S58
Kelly K, Crowley J, Bunn PA, Presant CA, Grevstad PK, et al (2001) Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non-small cell lung cancer. J Clin Oncol 19:3210–321
Khayat D, Rixe O, Dr Brunet, Dr Goupil, Bugat R, Harousseau JL, Dr Ifrah, Puozzo C (2004) Pharmacokinetic linearity of IV vinorelbine from an intra-patient dose escalation study design. Cancer Chemother Pharmacol 54(3):193–205
Le Chevalier T (1994) Randomized study of vinorelbine and cisplatin versus vindesine and cisplating versus vinorelbine alone in advanced non-small cell lung cancer: results of a European multicenter trial including 612 patients. J Clin Oncol 12:360–367
Liu G, Franssen E, Fitch MI, Warner E (1997) Patient preferences for oral versus intravenous palliative chemotherapy. J Clin Oncol 15(1):110–115
Lush RM, McCune JS, Tetteh L, Thompson JA, Mahany JJ, Garland L, Suttle AB, Sullivan DM (2005) The absolute bioavailability of oral vinorelbine in patients with solid tumors. Cancer Chemother Pharmacol 56(6):578–584
Marty M, Fumoleau P, Adenis A, Rousseau Y, Merrouche Y, Robinet G, Senac I, Puozzo C (2001) Oral vinorelbine pharmacokinetics and absolute bioavailability study in patients with solid tumors. Ann Oncol 12:1643–1649
Nguyen L, Tranchant B, Puozzo C, Variol P (2002) Population pharmacokinetics model and limited sampling strategy for intravenous vinorelbine derived from phase I clinical trials. Br J Clin Pharmacol 53:459–468
Puozzo C (2006) Can similar oral blood exposures between studies result in a different bioavailability? Cancer Chemother Pharmacol 58:838–841
Ramlau R, Hausen O, Wagner L et al (2003) A full Navelbine oral treatment in combination with cisplatin followed by NVB oral single agent as consolidation therapy in NSCLC. Eur J Cancer S247 (abst. 823)
Van Heugen JC, De Graeve J, Zorza G, Puozzo C (2001) New sensitive liquid chromatography method coupled with tandem mass spectrometric detection for the clinical analysis of vinorelbine and its metabolites in blood, plasma, urine and faeces. J Chromatogr 926:11–20
Wozniak AJ, Crowley JJ, Balcerzak SP et al (1998) Randomized trial comparing cisplatin with cisplatin versus cisplatin plus vinorelbine in the treatment of advanced non-small cell lung cancer: a Southwest Oncology Group study. J Clin Oncol 16(7):2459–2465
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bourgeois, H., Vermorken, J., Dark, G. et al. Evaluation of oral versus intravenous dose of vinorelbine to achieve equivalent blood exposures in patients with solid tumours. Cancer Chemother Pharmacol 60, 407–413 (2007). https://doi.org/10.1007/s00280-007-0510-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-007-0510-z