Abstract
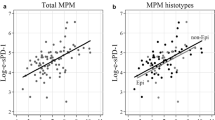
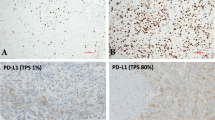
CTLA-4 function as a negative regulator of T cell-mediated immune response is well established, whereas much less is known about the immunoregulatory role of its soluble isoform (sCTLA-4). No data are available on CTLA-4 expression and prognostic impact in malignant pleural mesothelioma (MPM). We investigated, by immunohistochemistry, CTLA-4 expression in tumor tissues and, by ELISA, sCTLA-4 levels in sera and matched pleural effusions from 45 MPM patients. Prognostic effect of CTLA-4 expression on overall survival (OS) was assessed through Cox regression and prognostic significance expressed as death rate ratio (HR). We found that 56.0 % of MPM tissues expressed CTLA-4 with variable intensity and percentage of positive cells estimated by the immunoreactive score. sCTLA-4 levels were significantly higher in sera (S-sCTLA-4) than in pleural effusions (PE-sCTLA-4) (geometric mean ratio = 2.70, P value = 0.020). CTLA-4 expression at the tissue level was higher in the epithelioid histological subtype than in the sarcomatoid, whereas at the serum level, it was higher in the sarcomatoid subtype. A homogeneous favorable prognostic effect was found for CTLA-4 overexpression in tissue, serum and pleural effusion. Interestingly, only the PE-sCTLA-4 was found to be a statistically significant positive prognostic factor (HR = 0.37, 95 % CI = 0.18–0.77, P value = 0.007). Indeed, PE-sCTLA-4 correlated with CTLA-4 expression in tissues, whereas this latter expression showed a weak association with OS. To confirm our findings, further experimental evidences obtained from a larger cohort of MPM patients are required. However, our results would indicate a positive correlation of PE-sCTLA-4 levels and OS in MPM patients.



Similar content being viewed by others
Abbreviations
- APC:
-
Antigen-presenting cells
- BNG:
-
Pulmonary benign disease
- CI:
-
Confidence intervals
- CTLA-4:
-
Cytotoxic T lymphocyte antigen-4
- FFPE:
-
Formalin-fixed paraffin embedded
- GM:
-
Geometric mean
- HR:
-
Hazard ratio
- IHC:
-
Immunohistochemistry
- IRS:
-
Immunoreactive score
- mCTLA-4:
-
Membrane CTLA-4
- MPM:
-
Malignant pleural mesothelioma
- OS:
-
Overall survival
- PE:
-
Pleural effusion
- PE-sCTLA-4:
-
Soluble CTLA-4 in PE
- sCTLA-4:
-
Soluble CTLA-4
- S-sCTLA-4:
-
Soluble CTLA-4 in serum
- Teffs:
-
Effector T cells
- Tregs:
-
Regulatory T cells
References
Robinson BW, Lake RA (2005) Advances in malignant mesothelioma. N Engl J Med 353:1591–1603
Porpodis K, Zarogoulidis P, Boutsikou E, Papaioannou A, Machairiotis N, Tsakiridis K, Katsikogiannis N, Zaric B, Perin B, Huang H et al (2013) Malignant pleural mesothelioma: current and future perspectives. J Thorac Dis 5(Suppl 4):S397–S406
Ismail-Khan R, Robinson LA, Williams CC Jr, Garrett CR, Bepler G, Simon GR (2006) Malignant pleural mesothelioma: a comprehensive review. Cancer Control 13:255–263
Remon J, Reguart N, Corral J, Lianes P (2015) Malignant pleural mesothelioma: new hope in the horizon with novel therapeutic strategies. Cancer Treat Rev 41:27–34
Maker AV, Attia P, Rosenberg SA (2005) Analysis of the cellular mechanism of antitumor responses and autoimmunity in patients treated with CTLA-4 blockade. J Immunol 175:7746–7754
Calabrò L, Morra A, Fonsatti E, Cutaia O, Amato G, Giannarelli D, Di Giacomo AM, Danielli R, Altomonte M, Mutti L, Maio M (2013) Tremelimumab for patients with chemotherapy-resistant advanced malignant mesothelioma: an open-label, single-arm, phase 2 trial. Lancet Oncol 14:1104–1111
Calabrò L, Morra A, Fonsatti E, Cutaia O, Fazio C, Annesi D, Lenoci M, Amato G, Danielli R, Altomonte M et al (2015) Efficacy and safety of an intensified schedule of tremelimumab for chemotherapy-resistant malignant mesothelioma: an open-label, single-arm, phase 2 study. Lancet Respir Med 3:301–309
Walker LS, Sansom DM (2011) The emerging role of CTLA-4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunol 11:852–863
Krummel MF, Allison JP (1996) CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med 183:2533–2540
Brunner MC, Chambers CA, Chan FK, Hanke J, Winoto A, Allison JP (1999) CTLA-4-mediated inhibition of early events of T cell proliferation. J Immunol 162:5813–5820
Engelhardt JJ, Sullivan TJ, Allison JP (2006) CTLA-4 overexpression inhibits T cell responses through a CD28-B7-dependent mechanism. J Immunol 177:1052–1061
Zheng Y, Manzotti CN, Burke F, Dussably L, Qureshi O, Walker LS, Sansom DM (2008) Acquisition of suppressive function by activated human CD4 + CD25- T cells is associated with the expression of CTLA-4 not FoxP3. J Immunol 181:1683–1691
Oaks MK, Hallett KM (2000) Cutting edge: a soluble form of CTLA-4 in patients with autoimmune thyroid disease. J Immunol 164:5015–5018
Ward FJ, Dahal LN, Wijesekera SK, Abdul-Jawad SK, Kaewarpai T, Xu H, Vickers MA, Barker RN (2013) The soluble isoform of CTLA-4 as a regulator of T-cell responses. Eur J Immunol 43:1274–1285
Saverino D, Brizzolara R, Simone R, Chiappori A, Milintenda-Floriani F, Pesce G, Bagnasco M (2007) Soluble CTLA-4 in autoimmune thyroid diseases: relationship with clinical status and possible role in the immune response dysregulation. Clin Immunol 123:190–198
Simone R, Brizzolara R, Chiappori A, Milintenda-Floriani F, Natale C, Greco L, Schiavo M, Bagnasco M, Pesce G, Saverino D (2009) A functional soluble form of CTLA-4 is present in the serum of celiac patients and correlates with mucosal injury. Int Immunol 21:1037–1045
Ueda H, Howson JM, Esposito L, Heward J, Snook H, Chamberlain G, Rainbow DB, Hunter KM, Smith AN, Di Genova G et al (2003) Association of the T-cell regulatory gene CTLA-4 with susceptibility to autoimmune disease. Nature 423:506–511
Simone R, Tenca C, Fais F, Luciani M, De Rossi G, Pesce G, Bagnasco M, Saverino D (2012) A soluble form of CTLA-4 is present in paediatric patients with acute lymphoblastic leukaemia and correlates with CD1d + expression. PLoS One 7(9):e44654
Erfani N, Razmkhah M, Ghaderi A (2010) Circulating soluble CTLA-4 (sCTLA-4) is elevated in patients with breast cancer. Cancer Invest 28:828–832
Contardi E, Palmisano GL, Tazzari PL, Martelli AM, Falà F, Fabbi M, Kato T, Lucarelli E, Donati D, Polito L et al (2005) CTLA-4 is constitutively expressed on tumor cells and can trigger apoptosis upon ligand interaction. Int J Cancer 117:538–550
Laurent S, Queirolo P, Boero S, Salvi S, Piccioli P, Boccardo S, Minghelli S, Morabito A, Fontana V, Pietra G et al (2013) The engagement of CTLA-4 on primary melanoma cell lines induces antibody-dependent cellular cytotoxicity and TNF-α production. J Transl Med 11:108
Salvi S, Fontana V, Boccardo S, Merlo DF, Margallo E, Laurent S, Morabito A, Rijavec E, Dal Bello MG, Mora M, Ratto GB, Grossi F, Truini M, Pistillo MP (2012) Evaluation of CTLA-4 expression and relevance as a novel prognostic factor in patients with non-small cell lung cancer. Cancer Immunol Immunother 61:1463–7214
Shah KV, Chien AJ, Yee C, Moon RT (2008) CTLA-4 is a direct target of Wnt/beta-catenin signalling and is expressed in human melanoma tumors. J Invest Dermatol 128:2870–2879
Mao H, Zhang L, Yang Y, Zuo W, Bi Y, Gao W, Deng B, Sun J, Shao Q, Qu X (2010) New insights of CTLA-4 into its biological function in breast cancer. Curr Cancer Drug Targets 10:728–736
Antczak A, Pastuszak-Lewandoska D, Górski P, Domańska D, Migdalska-Sęk M, Czarnecka K, Nawrot E, Kordiak J, Brzeziańska E (2013) CTLA-4 expression and polymorphisms in lung tissue of patients with diagnosed non-small-cell lung cancer. Biomed Res Int 2013:576486
Marubini E, Valsecchi MG (2004) Analysing survival data from clinical trials and observational studies. Wiley & Sons, New York
Liang H, Zou G (2008) Improved AIC selection strategy for survival analysis. Comput Stat Data Anal 52:2538–2548
DiCiccio TJ, Efron B (1996) Bootstrap confidence intervals. Stat Sci 11:189–228
Leung HT, Bradshaw J, Cleaveland JS, Linsley PS (1995) Cytotoxic T lymphocyte-associated molecule-4, a high-avidity receptor for CD80 and CD86, contains an intracellular localization motif in its cytoplasmic tail. J Biol Chem 270:25107–25114
Tai X, Van Laethem F, Pobezinsky L, Guinter T, Sharrow SO, Adams A, Granger L, Kruhlak M, Lindsten T, Thompson CB et al (2012) Basis of CTLA-4 function in regulatory and conventional CD4(+) T cells. Blood 119:5155–5163
Valdés L, San-José E, Estévez JC, González-Barcala FJ, Alvarez-Dobaño JM, Golpe A, Valle JM, Penela P, Vizcaíno L, Pose A (2010) Cholesterol in pleural exudates depends mainly on increased capillary permeability. Transl Res 155:178–184
Chen YQ, Shi HZ, Qin XJ, Mo WN, Liang XD, Huang ZX, Yang HB, Wu C (2005) CD4 + CD25 + regulatory T lymphocytes in malignant pleural effusion. Am J Respir Crit Care Med 172:1434–1439
Esposito L, Hunter KM, Clark J, Rainbow DB, Stevens H, Denesha J, Duley S, Dawson S, Coleman G, Nutland S et al (2014) Investigation of soluble and transmembrane CTLA-4 isoforms in serum and microvesicles. J Immunol 193:889–900
Cao J, Zhang L, Huang S, Chen P, Zou L, Chen H, Xiang Y, Lai X, Ren G (2011) Aberrant production of soluble co-stimulatory molecules CTLA-4 and CD28 in patients with chronic hepatitis B. Microb Pathog 51:262–267
Steiner K, Moosig F, Csernok E, Selleng K, Gross WL, Fleischer B, Bröker BM (2001) Increased expression of CTLA-4 (CD152) by T and B lymphocytes in Wegener’s granulomatosis. Clin Exp Immunol 126:143–150
Shen Y, Yang T, Wang T, Chen L, Wen F (2013) Elevated circulating cytotoxic T lymphocyte-associated antigen-4 levels are related to lung function and inflammation in chronic obstructive pulmonary disease. Respirology 18:885–886
Musk AW, Olsen N, Alfonso H, Reid A, Mina R, Franklin P, Sleith J, Hammond N, Threlfall T, Shilkin KB, de Klerk NH (2011) Predicting survival in malignant mesothelioma. Eur Respir J 38:1420–1424
Guazzelli A, Hussain M, Krstic-Demonacos M, Mutti L (2015) Tremelimumab for the treatment of malignant mesothelioma. Expert Opin Biol Ther 15:1819–1829
Ward FJ, Dahal LN, Khanolkar RC, Shankar SP, Barker RN (2014) Targeting the alternatively spliced soluble isoform of CTLA-4: prospects for immunotherapy? Immunotherapy 6:1073–1084
Acknowledgments
Supported in part by Grants from the Italian Ministry of Health (5 × 1000 funds 2011), AIL Sezione La Spezia and Fondazione Umberto Veronesi (Grant 2013), Milan, Italy. The authors would like to thank Dr. Roberto Benelli for image analysis and graphical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
S. Roncella and S. Laurent contributed equally to this work.
Rights and permissions
About this article
Cite this article
Roncella, S., Laurent, S., Fontana, V. et al. CTLA-4 in mesothelioma patients: tissue expression, body fluid levels and possible relevance as a prognostic factor. Cancer Immunol Immunother 65, 909–917 (2016). https://doi.org/10.1007/s00262-016-1844-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-016-1844-3