Abstract
Purpose
This study aimed to investigate the diagnostic performance of quantitative DCE-MRI for characterizing ovarian tumors.
Methods
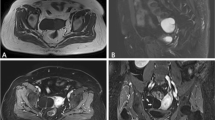
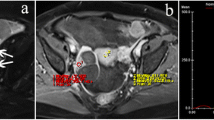
We prospectively assessed the differences of quantitative DCE-MRI parameters (Ktrans, kep, and ve) among 15 benign, 28 borderline, and 66 malignant ovarian tumors; and between type I (n = 28) and type II (n = 29) of epithelial ovarian carcinomas (EOCs). DCE-MRI data were analyzed using whole solid tumor volume region of interest (ROI) method, and quantitative parameters were calculated based on a modified Tofts model. The non-parametric Kruskal–Wallis test, Mann–Whitney U test, Pearson’s chi-square test, intraclass correlation coefficient (ICC), variance test, and receiver operating characteristic curves (ROC) were used for statistical analysis.
Results
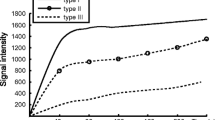
The largest Ktrans and kep values were observed in ovarian malignant tumors, followed by borderline and benign tumors (all P < 0.001). Kep was the better parameter for differentiating benign tumors from borderline and malignant tumors, with a sensitivity of 89.3% and 95.5%, a specificity of 86.7% and 100%, an accuracy of 88.4% and 96.3%, and an area under the curve (AUC) of 0.94 and 0.992, respectively, whereas Ktrans was better for differentiating borderline from malignant tumors with a sensitivity of 60.7%, a specificity of 78.8%, an accuracy of 73.4%, and an AUC of 0.743. In addition, a combination with kep could further improve the sensitivity to 78.9%. The median Ktrans and kep values were significantly higher in type II than in type I EOCs.
Conclusion
DCE-MRI with volume quantification is a technically feasible method, and can be used for the differentiation of ovarian tumors and for discriminating between type I and type II EOCs.





Similar content being viewed by others
References
Kurman RJ, Carcangiu ML, Herrington CS, Young RH (2014) WHO classification of tumours of female reproductive organs. Lyon: IARC
Morgan RJ, Armstrong DK, Alvarez RD, et al. (2016) Ovarian cancer, version 1.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 14:1134–1163
Kurman RJ, Shih IM (2010) The origin and pathogenesis of epithelial ovarian cancer – a proposed unifying theory. Am J Surg Pathol 34:433–443
Satoh T, Hatae M, Watanabe Y, et al. (2010) Outcomes of fertility-sparing surgery for stage I epithelial ovarian cancer: a proposal for patient selection. J Clin Oncol 28:1727–1732
Song T, Choi CH, Kim HJ, et al. (2011) Accuracy of frozen section diagnosis of borderline ovarian tumors. Gynecol Oncol 122:127–131
Bazot M, Nassar-Slaba J, Thomassin-Naggara I, et al. (2006) MR imaging compared with intraoperative frozen-section examination for the diagnosis of adnexal tumors: correlation with final histology. Eur Radiol 16:2687–2699
Vargas HA, Barrett T, Sala E (2013) MRI of ovarian masses. J Magn Reson Imaging 37:265–281
García-Figueiras R, Padhani AR, Beer AJ, et al. (2015) Imaging of tumor angiogenesis for radiologists—Part 2: Clinical utility. Curr Probl Diagn Radiol 44(5):425–436
Zahra MA, Hollingsworth KG, Sala E, Lomas DJ, Tan LT (2007) Dynamic contrast-enhanced MRI as a predictor of tumour response to radiotherapy. Lancet Oncol 8(1):63–74
O’Connor JP, Jackson A, Parker GJ, Roberts C, Jayson GC (2012) Dynamic contrast-enhanced MR in clinical trials of antivascular therapies. Nat Rev Clin Oncol 9(3):167–177
Miller JC, Pien HH, Sahani D, Sorensen AG, Thrall JH (2005) Imaging angiogenesis: applications and potential for drug development. J Natl Cancer Inst 97(3):172–187
Kazerooni AF, Malek M, Haghighatkhah H, et al. (2017) Semiquantitative dynamic contrast-enhanced MRI for accurate classification of complex adnexal masses. J Magn Reson Imaging 45(2):418–427
Mansour SM, Saraya S, EI-Faissal Y (2015) Semi-quantitative contrast-enhanced MR analysis of indeterminate ovarian tumours: when to say malignancy? Br J Radiol 1053(88):20150099. https://doi.org/10.1259/bjr.20150099
Thomassin-Naggara I, Bazot M, Daraï E, et al. (2008) Epithelial ovarian tumors: value of dynamic contrast-enhanced MR imaging and correlation with tumor angiogenesis. Radiology 248(1):148–159
Thomassin-Naggara I, Daraï E, Cuenod CA, et al. (2008) Dynamic contrast-enhanced magnetic resonance imaging: a useful tool for characterizing ovarian epithelial tumors. J Magn Reson Imaging 28(1):111–120
Li HM, Qiang JW, Ma FH, Zhao SH (2017) The value of dynamic contrast-enhanced MRI in characterizing complex ovarian tumors. J Ovarian Res 10(1):4. https://doi.org/10.1186/s13048-017-0302-y
Dilks P, Narayanan P, Reznek R, Sahdev A, Rockall A (2010) Can quantitative dynamic contrast-enhanced MRI independently characterize an ovarian mass? Eur Radiol 20(9):2176–2183
Bernardin L, Dilks P, Liyanage S, et al. (2012) Effectiveness of semi-quantitative multiphase dynamic contrast-enhanced MRI as a predictor of malignancy in complex adnexal masses: radiological and pathological correlation. Eur Radiol 22(4):880–890
Patel N, Harris AL, Gleeson FV, Vallis KA (2012) Clinical imaging of tumor angiogenesis. Future Oncol 8(11):1443–1459
Carter JS, Koopmeiners JS, Kuehn-Hajder JE, et al. (2013) Quantitative multiparametric MRI of ovarian cancer. J Magn Reson Imaging 38(6):1501–1509
Priest AN, Gill AB, Kataoka M, et al. (2010) Dynamic contrast-enhanced MRI in ovarian cancer: Initial experience at 3 tesla in primary and metastatic disease. Magn Reson Med 63(4):1044–1049
Sala E, Kataoka MY, Priest AN, et al. (2012) Advanced ovarian cancer: multiparametric MR imaging demonstrates response- and metastasis-specific effects. Radiology 263(1):149–159
Thomassin-Naggara I, Balvay D, Aubert E, et al. (2012) Quantitative dynamic contrast-enhanced MR imaging analysis of complex adnexal masses: a preliminary study. Eur Radiol 22(4):738–745
Thomassin-Naggara I, Soualhi N, Balvay D, Darai E, Cuenod CA (2017) Quantifying tumor vascular heterogeneity with DCE-MRI in complex adnexal massed: a preliminary study. J Magn Resson Imaging 46(6):1776–1785
Wang HY, Su ZH, Xu X, et al. (2016) Dynamic contrast-enhanced MR imaging in renal cell carcinoma: reproducibility of histogram analysis on pharmacokinetic parameters. Sci Rep 6:29146. https://doi.org/10.1038/srep29146
García-Figueiras R, Padhani AR, Beer AJ, et al. (2015) Imaging of tumor angiogenesis for radiologists—Part 1: biological and technical basis. Curr Probl Diagn Radiol 44(5):407–424
Venkatasubramanian R, Arenas RB, Henson MA, Forbes NS (2010) Mechanistic modelling of dynamic MRI data predicts that tumor heterogeneity decreases therapeutic response. Br J Cancer 103(4):486–497
Liu C, Wang K, Chan Q, et al. (2016) Intravoxel incoherent motion MR imaging for breast lesions: comparison and correlation with pharmacokinetic evaluation from dynamic contrast-enhanced MR imaging. Eur Radiol 26(11):3888–3898
Yuan M, Zhang YD, Zhu C, et al. (2016) Comparison of intravoxel incoherent motion diffusion-weighted MR imaging with dynamic contrast-enhanced MRI for differentiating lung cancer from benign solitary pulmonary lesions. J Magn Reson Imaging 43(3):669–679
Xian J, Du H, Wang X, et al. (2014) Feasibility and value of quantitative dynamic contrast enhancement MR imaging in the evaluation of sinonasal tumors. Chin Med J (Engl) 127(12):2259–2264
Shin JK, Kim JY (2017) Dynamic contrast-enhanced and diffusion-weighted MRI of estrogen receptor-positive invasive breast cancers: associations between quantitative MR parameters and Ki-67 proliferation status. J Magn Reson Imaging 45(1):94–102
Zhang N, Zhang L, Qiu B, et al. (2012) Correlation of volume transfer coefficient Ktrans with histopathologic grades of gliomas. J Magn Reson Imaging 36(2):355–363
Mitchell CL, O’Connor JP, Jackson A, et al. (2010) Identification of early predictive imaging biomarkers and their relationship to serological angiogenic markers in patients with ovarian cancer with residual disease following cytotoxic therapy. Ann Oncol 21(10):1982–1989
Szubert S, Moszynski R, Michalak S, et al. (2016) The associations between serum VEGF, bFGF and endoglin levels with microvessel density and expression of proangiogenic factors in malignant and benign ovarian tumors. Microvasc Res 107:91–96
Thukral A, Thomasson DM, Chow CK, et al. (2007) Inflammatory breast cancer: dynamic contrast-enhanced MR in patients receiving bevacizumab-initial experience. Radiology 244(3):727–735
Inven M, Reerink O, Philippens ME (2015) Dynamic contrast MR imaging for rectal cancer response assessment after neo-adjuvant chemotherapy. J Magn Reson Imaging 41(6):1646–1653
Sahani DV, Jiang T, Hayano K, et al. (2013) Magnetic resonance imaging biomarkers in hepatocellular carcinoma: association with response and circulating biomarkers after sunitinib therapy. J Hematol Oncol 6:51
Yang J, Kim JH, Im GH, et al. (2011) Evaluation of antiangiogenic effects of a new synthetic candidate drug KR-31831 on xenografted ovarian carcinoma using dynamic contrast enhanced MRI. Korean J Radiol 12(5):602–610
Li X, Cai Y, Moloney B, et al. (2016) Relative sensitivities of DCE-MRI pharmacokinetic parameters to arterial input function (AIF) scaling. J Magn Reson 269:104–112
Yeo DM, Oh SN, Jung CK, et al. (2015) Correlation of dynamic contrast-enhanced MRI perfusion parameters with angiogenesis and biologic aggressiveness of rectal cancer: preliminary results. J Magn Reson Imaging 41(2):474–480
Yi B, Kang DK, Yoon D, et al. (2014) Is there any correlation between model-based perfusion parameters and model-free parameters of time-signal intensity curve on dynamic contrast enhanced MRI in breast cancer patients? Eur Radiol 24(5):1089–1096
Jackson A, O’Connor JP, Parker GJ, Jayson GC (2007) Imaging tumor vascular heterogeneity and angiogenesis using dynamic contrast-enhanced magnetic resonance imaging. Clin Cancer Res 13(12):3449–3459
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This study was funded by National Natural Science Foundation of China (Nos. 81471628 and 81501439), Nantong Municipal Commission of Health and Family Planning Science Foundation for Youth (No. WQ2016065), and Shanghai Municipal Commission of Health and Family Planning (Nos. 2013ZYJB0201, 2013SY075, and ZK2015A05).
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with 1964 Helsinki declaration and its later amendments or comparable ethical standard.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Li, Hm., Feng, F., Qiang, Jw. et al. Quantitative dynamic contrast-enhanced MR imaging for differentiating benign, borderline, and malignant ovarian tumors. Abdom Radiol 43, 3132–3141 (2018). https://doi.org/10.1007/s00261-018-1569-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-018-1569-1