Abstract
Purpose
This is a radiomics study investigating the ability of texture analysis of MRF maps to improve differentiation between intra-axial adult brain tumors and to predict survival in the glioblastoma cohort.
Methods
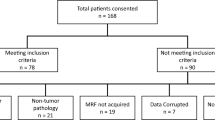
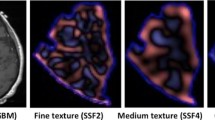
Magnetic resonance fingerprinting (MRF) acquisition was performed on 31 patients across 3 groups: 17 glioblastomas, 6 low-grade gliomas, and 8 metastases. Using regions of interest for the solid tumor and peritumoral white matter on T1 and T2 maps, second-order texture features were calculated from gray-level co-occurrence matrices and gray-level run length matrices. Selected features were compared across the three tumor groups using Wilcoxon rank-sum test. Receiver operating characteristic curve analysis was performed for each feature. Kaplan-Meier method was used for survival analysis with log rank tests.
Results
Low-grade gliomas and glioblastomas had significantly higher run percentage, run entropy, and information measure of correlation 1 on T1 than metastases (p < 0.017). The best separation of all three tumor types was seen utilizing inverse difference normalized and homogeneity values for peritumoral white matter in both T1 and T2 maps (p < 0.017). In solid tumor T2 maps, lower values in entropy and higher values of maximum probability and high-gray run emphasis were associated with longer survival in glioblastoma patients (p < 0.05). Several texture features were associated with longer survival in glioblastoma patients on peritumoral white matter T1 maps (p < 0.05).
Conclusion
Texture analysis of MRF-derived maps can improve our ability to differentiate common adult brain tumors by characterizing tumor heterogeneity, and may have a role in predicting outcomes in patients with glioblastoma.




Similar content being viewed by others
Data availability
Upon reasonable request.
Abbreviations
- AUC:
-
area under the curve
- GLCM:
-
gray-level co-occurrence matrix
- IMC1:
-
information measure of correlation 1
- LGG:
-
lower grade glioma
- MR:
-
magnetic resonance
- MRF:
-
magnetic resonance fingerprinting
- OS:
-
overall survival
- ROC:
-
receiver operating characteristic
- ROI:
-
region of interest
- ST:
-
solid tumor
- PW:
-
peritumoral white matter
References
Sottoriva A, Spiteri I, Piccirillo SGM, Touloumis A, Collins VP, Marioni JC, et al. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc Natl Acad Sci U S A. 2013;110:4009–14.
Parker NR, Khong P, Parkinson JF, Howell VM, Wheeler HR. Molecular heterogeneity in glioblastoma: potential clinical implications. Front Oncol. 2015;5:55.
Molina D, Pérez-Beteta J, Luque B, Arregui E, Calvo M, Borrás JM, et al. Tumour heterogeneity in glioblastoma assessed by MRI texture analysis: a potential marker of survival. Br J Radiol [Internet]. [cited 2020 Apr 15];89. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5124892/
Hegi ME, Diserens A-C, Gorlia T, Hamou M-F, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003.
Colman H, Zhang L, Sulman EP, McDonald JM, Shooshtari NL, Rivera A, et al. A multigene predictor of outcome in glioblastoma. Neuro-Oncol. 2010;12:49–57.
Aibaidula A, Chan AK-Y, Shi Z, Li Y, Zhang R, Yang R, et al. Adult IDH wild-type lower-grade gliomas should be further stratified. Neuro-Oncol. 2017;19:1327–37.
Chang SM, Cahill DP, Aldape KD, Mehta MP. Treatment of adult lower-grade glioma in the era of genomic medicine. Am Soc Clin Oncol Educ Book Am Soc Clin Oncol Annu Meet. 2016;35:75–81.
Miller JA, Bennett EE, Xiao R, Kotecha R, Chao ST, Vogelbaum MA, et al. Association between radiation necrosis and tumor biology after stereotactic radiosurgery for brain metastasis. Int J Radiat Oncol Biol Phys. 2016;96:1060–9.
Abrol S, Kotrotsou A, Salem A, Zinn PO, Colen RR. Radiomic phenotyping in brain cancer to unravel hidden information in medical images. Top Magn Reson Imaging TMRI. 2017;26:43–53.
Wibmer A, Hricak H, Gondo T, Matsumoto K, Veeraraghavan H, Fehr D, et al. Haralick texture analysis of prostate MRI: utility for differentiating non-cancerous prostate from prostate cancer and differentiating prostate cancers with different Gleason scores. Eur Radiol. 2015;25:2840–50.
Aerts HJWL, Velazquez ER, Leijenaar RTH, Parmar C, Grossmann P, Carvalho S, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014;5:4006.
Grove O, Berglund AE, Schabath MB, Aerts HJWL, Dekker A, Wang H, et al. Quantitative computed tomographic descriptors associate tumor shape complexity and intratumor heterogeneity with prognosis in lung adenocarcinoma. PLoS One. 2015;10:e0118261.
Kuo MD, Gollub J, Sirlin CB, Ooi C, Chen X. Radiogenomic analysis to identify imaging phenotypes associated with drug response gene expression programs in hepatocellular carcinoma. J Vasc Interv Radiol JVIR. 2007;18:821–31.
Chen Y-H, Lue K-H, Chu S-C, Chang B-S, Wang L-Y, Liu D-W, et al. Combining the radiomic features and traditional parameters of 18F-FDG PET with clinical profiles to improve prognostic stratification in patients with esophageal squamous cell carcinoma treated with neoadjuvant chemoradiotherapy and surgery. Ann Nucl Med. 2019;33:657–70.
Cozzi L, Franzese C, Fogliata A, Franceschini D, Navarria P, Tomatis S, et al. Predicting survival and local control after radiochemotherapy in locally advanced head and neck cancer by means of computed tomography based radiomics. Strahlenther Onkol. 2019;195:805–18.
Leithner D, Bernard-Davila B, Martinez DF, Horvat JV, Jochelson MS, Marino MA, et al. Radiomic signatures derived from diffusion-weighted imaging for the assessment of breast Cancer receptor status and molecular subtypes. Mol Imaging Biol. 2020;22:453–61.
Abdollahi H, Tanha K, Mofid B, Razzaghdoust A, Saadipoor A, Khalafi L, et al. MRI radiomic analysis of IMRT-induced bladder wall changes in prostate cancer patients: a relationship with radiation dose and toxicity. J Med Imaging Radiat Sci. 2019;50:252–60.
Wang H, Nie P, Wang Y, Xu W, Duan S, Chen H, et al. Radiomics nomogram for differentiating between benign and malignant soft-tissue masses of the extremities. J Magn Reson Imaging. 2020;51:155–63.
Herlidou S, Rolland Y, Bansard JY, Le Rumeur E, de Certaines JD. Comparison of automated and visual texture analysis in MRI: characterization of normal and diseased skeletal muscle. Magn Reson Imaging. 1999;17:1393–7.
Haralick RM, Shanmugam K, Dinstein I. Textural features for image classification. IEEE Trans Syst Man Cybern. 1973;SMC-3:610–21.
Kassner A, Thornhill RE. Texture analysis: a review of neurologic MR imaging applications. AJNR Am J Neuroradiol. 2010;31:809–16.
Haralick RM. Statistical and structural approaches to texture. Proc IEEE. 1979;67:786–804.
Chaddad A, Desrosiers C, Toews M. Radiomic analysis of multi-contrast brain MRI for the prediction of survival in patients with glioblastoma multiforme. Conf Proc IEEE Eng Med Biol Soc. 2016;2016:4035–8.
Liu Y, Xu X, Yin L, Zhang X, Li L, Lu H. Relationship between glioblastoma heterogeneity and survival time: an MR imaging texture analysis. AJNR Am J Neuroradiol. 2017;38:1695–701.
Chaddad A, Daniel P, Desrosiers C, Toews M, Abdulkarim B. Novel radiomic features based on joint intensity matrices for predicting glioblastoma patient survival time. IEEE J Biomed Health Inform. 2019;23:795–804.
Chaddad A, Sabri S, Niazi T, Abdulkarim B. Prediction of survival with multi-scale radiomic analysis in glioblastoma patients. Med Biol Eng Comput. 2018;56:2287–300.
Prasanna P, Patel J, Partovi S, Madabhushi A, Tiwari P. Radiomic features from the peritumoral brain parenchyma on treatment-naïve multi-parametric MR imaging predict long versus short-term survival in glioblastoma multiforme: preliminary findings. Eur Radiol. 2017;27:4188–97.
Liu X, Li Y, Li S, Fan X, Sun Z, Yang Z, et al. IDH mutation-specific radiomic signature in lower-grade gliomas. Aging. 2019;11:673–96.
Yu J, Shi Z, Lian Y, Li Z, Liu T, Gao Y, et al. Noninvasive IDH1 mutation estimation based on a quantitative radiomics approach for grade II glioma. Eur Radiol. 2017;27:3509–22.
Ryu YJ, Choi SH, Park SJ, Yun TJ, Kim J-H, Sohn C-H. Glioma: application of whole-tumor texture analysis of diffusion-weighted imaging for the evaluation of tumor heterogeneity. PLoS ONE [Internet]. 2014 [cited 2020 Mar 3];9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4182447/
O’Connor JPB, Aboagye EO, Adams JE, Aerts HJWL, Barrington SF, Beer AJ, et al. Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol. 2017;14:169–86.
Haubold J, Demircioglu A, Gratz M, Glas M, Wrede K, Sure U, et al. Non-invasive tumor decoding and phenotyping of cerebral gliomas utilizing multiparametric 18F-FET PET-MRI and MR fingerprinting. Eur J Nucl Med Mol Imaging [Internet]. 2019 [cited 2020 Apr 15]; Available from: https://doi.org/10.1007/s00259-019-04602-2.
Wang S, Meng M, Zhang X, Wu C, Wang R, Wu J, et al. Texture analysis of diffusion weighted imaging for the evaluation of glioma heterogeneity based on different regions of interest. Oncol Lett. 2018;15:7297–304.
Ma D, Gulani V, Seiberlich N, Liu K, Sunshine JL, Duerk JL, et al. Magnetic resonance fingerprinting. Nature. 2013;495:187–92.
Badve C, Yu A, Dastmalchian S, Rogers M, Ma D, Jiang Y, et al. MR fingerprinting of adult brain tumors: initial experience. Am J Neuroradiol. 2017;38:492–9.
Assefa D, Keller H, Ménard C, Laperriere N, Ferrari RJ, Yeung I. Robust texture features for response monitoring of glioblastoma multiforme on T1-weighted and T2-FLAIR MR images: a preliminary investigation in terms of identification and segmentation. Med Phys. 2010;37:1722–36.
Mahmoud-Ghoneim D, Alkaabi MK, de Certaines JD, Goettsche F-M. The impact of image dynamic range on texture classification of brain white matter. BMC Med Imaging. 2008;8:18.
Sharma H. Multiparametric imaging and MR image texture analysis in brain tumors. Electron Thesis Diss Repos [Internet]. 2014; Available from: https://ir.lib.uwo.ca/etd/1967
DrS GN, Shobha G. Statistical texture analysis proceedings of World Academy of Science. Eng Technol. 2008;36:2070–3740.
Conners RW, Harlow CA. A theoretical comparison of texture algorithms. IEEE Trans Pattern Anal Mach Intell. 1980;2:204–22.
Hu LS, Ning S, Eschbacher JM, Gaw N, Dueck AC, Smith KA, et al. Multi-parametric MRI and texture analysis to visualize spatial histologic heterogeneity and tumor extent in glioblastoma. PLoS One. 2015;10:e0141506.
Arya R, Singh N, Agrawal RK. A novel combination of second-order statistical features and segmentation using multi-layer superpixels for salient object detection. Appl Intell. 2017;46:254–71.
Lupo JM, Cha S, Chang SM, Nelson SJ. Dynamic susceptibility-weighted perfusion imaging of high-grade gliomas: characterization of spatial heterogeneity. AJNR Am J Neuroradiol. 2005;26:1446–54.
Hambardzumyan D, Bergers G. Glioblastoma: defining tumor niches. Trends Cancer. 2015;1:252–65.
Wagner M, Nafe R, Jurcoane A, Pilatus U, Franz K, Rieger J, et al. Heterogeneity in malignant gliomas: a magnetic resonance analysis of spatial distribution of metabolite changes and regional blood volume. J Neuro-Oncol. 2011;103:663–72.
Barajas RF, Phillips JJ, Parvataneni R, Molinaro A, Essock-Burns E, Bourne G, et al. Regional variation in histopathologic features of tumor specimens from treatment-naive glioblastoma correlates with anatomic and physiologic MR imaging. Neuro-Oncol. 2012;14:942–54.
Ji B, Wang S, Liu Z, Weinberg BD, Yang X, Liu T, et al. Revealing hemodynamic heterogeneity of gliomas based on signal profile features of dynamic susceptibility contrast-enhanced MRI. NeuroImage Clin. 2019;23:101864.
Deoni SCL. Quantitative relaxometry of the brain. Top Magn Reson Imaging TMRI. 2010;21:101–13.
Inam Ul Haq M. Texture analysis in the Logarithmic Image Processing (LIP) framework. 2013;
Chenevert TL, Stegman LD, Taylor JMG, Robertson PL, Greenberg HS, Rehemtulla A, et al. Diffusion magnetic resonance imaging: an early surrogate marker of therapeutic efficacy in brain tumors. JNCI J Natl Cancer Inst Oxford Academic. 2000;92:2029–36.
Mouthuy N, Cosnard G, Abarca-Quinones J, Michoux N. Multiparametric magnetic resonance imaging to differentiate high-grade gliomas and brain metastases. J Neuroradiol. 2012;39:301–7.
Funding
This work was supported by National Institutes of Health 1R01BB017219 award (Principal Investigator: Dr. Mark Griswold) and 1R01EB016728 award (Principal Investigators: Drs. Mark Griswold and Vikas Gulani). This project was also supported by the Clinical and Translational Science Collaborative (CTSC) of Cleveland which is funded by the National Institutes of Health (NIH), National Center for Advancing Translational Science (NCATS), Clinical and Translational Science Award (CTSA) grant, UL1TR002548 (Principal Investigator: Dr. Chaitra Badve). AES is supported by NIH CA217956, the Peter D Cristal Chair, the Center of Excellence for Translational Neuro-Oncology, the Gerald R. Kaufman Fund for Glioma Research at University Hospitals of Cleveland, the Kimble Family Foundation, and the Ferry Family Foundation at University Hospitals of Cleveland. The content is solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Contributions
All of the following authors have contributed substantially to the submitted work.
Sara Dastmalchian:
• Contributed equally to this work as: Ozden Kilinc.
• Substantial contributions to the conception and design of the work; the acquisition, analysis, and interpretation of data for the work;
• Drafting the manuscript and revising it critically for important intellectual content;
• Final approval of the version to be published;
• Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ozden Kilinc:
• Contributed equally to this work as: Sara Dastmalchian.
• Substantial contributions to the conception and design of the work; the acquisition, analysis, and interpretation of data for the work;
• Drafting the manuscript and revising it critically for important intellectual content;
• Final approval of the version to be published;
• Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Louisa Onyewadume:
• Substantial contributions to the analysis and interpretation of data for the work;
• Revising the manuscript critically for important intellectual content;
• Final approval of the version to be published;
• Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Charit Tippareddy:
• Substantial contributions to the analysis and interpretation of data for the work;
• Drafting the manuscript and revising it critically for important intellectual content;
• Final approval of the version to be published;
• Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Debra McGivney:
• Substantial contributions to the conception and design of the work, analysis, and interpretation of data for the work;
• Revising the manuscript critically for important intellectual content;
• Final approval of the version to be published;
• Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Dan Ma:
• Substantial contributions to the conception and design of the work; and the acquisition and analysis of data for the work;
• Revising the manuscript critically for important intellectual content;
• Final approval of the version to be published;
• Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Mark Griswold:
• Substantial contributions to the conception and design of the work; and the acquisition of data for the work;
• Revising the manuscript critically for important intellectual content;
• Final approval of the version to be published;
• Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Jeffrey Sunshine:
• Substantial contributions to the conception and design of the work;
• Revising the manuscript critically for important intellectual content;
• Final approval of the version to be published;
• Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Vikas Gulani:
• Substantial contributions to the conception and design of the work; and the acquisition of data for the work;
• Revising the manuscript critically for important intellectual content;
• Final approval of the version to be published;
• Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Jill S. Barnholtz-Sloan:
• Substantial contributions to the conception and design of the work; analysis, and interpretation of data for the work;
• Revising the manuscript critically for important intellectual content;
• Final approval of the version to be published;
• Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Andrew E. Sloan:
• Substantial contributions to the conception and design of the work; and the acquisition of data for the work;
• Revising the manuscript critically for important intellectual content;
• Final approval of the version to be published;
• Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Chaitra Badve:
• Substantial contributions to the conception and design of the work; the acquisition, analysis, and interpretation of data for the work;
• Drafting the manuscript and revising it critically for important intellectual content;
• Final approval of the version to be published;
• Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
Case Western Reserve University and University Hospitals receive research support from Siemens. Chaitra Badve, Dan Ma, Andrew E. Sloan, Jeffrey Sunshine, Mark Griswold, and Vikas Gulani have patent applications on MRF applications in brain tumors.
Sara Dastmalchian, Ozden Kilinc, Louisa Onyewadume, Charit Tippareddy, Debra McGivney, Jill Barnholtz-Sloan do not have any relevant conflicts of interest to disclose.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Code availability
NA
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Advanced Image Analyses (Radiomics and Artificial Intelligence).
Rights and permissions
About this article
Cite this article
Dastmalchian, S., Kilinc, O., Onyewadume, L. et al. Radiomic analysis of magnetic resonance fingerprinting in adult brain tumors. Eur J Nucl Med Mol Imaging 48, 683–693 (2021). https://doi.org/10.1007/s00259-020-05037-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-020-05037-w