Abstract
Purpose
Lymphovascular invasion (LVI) impairs surgical outcomes in lung adenocarcinoma (LAC) patients. Preoperative prediction of LVI is challenging by using traditional clinical and imaging parameters. The purpose of this study was to investigate the value of the radiomics nomogram integrating clinical factors, CT features, and maximum standardized uptake value (SUVmax) to predict LVI and outcome in LAC and to evaluate the additional value of the SUVmax to the PET/CT-based radiomics nomogram.
Methods
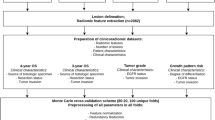
A total of 272 LAC patients (87 LVI-present LACs and 185 LVI-absent LACs) with PET/CT scans were retrospectively enrolled, and 160 patients with SUVmax ≥ 2.5 of them were used for PET radiomics analysis. Clinical data and CT features were analyzed to select independent LVI predictors. The performance of the independent LVI predictors and SUVmax was evaluated. Two-dimensional (2D) and three-dimensional (3D) CT radiomics signatures (RSs) and PET-RS were constructed with the least absolute shrinkage and selection operator algorithm and radiomics scores (Rad-scores) were calculated. The radiomics nomograms, incorporating Rad-score and independent clinical and CT factors, with SUVmax (RNWS) or without SUVmax (RNWOS) were built. The performance of the models was assessed with respect to calibration, discrimination, and clinical usefulness. All the clinical, PET/CT, pathologic, therapeutic, and radiomics parameters were assessed to identify independent predictors of progression-free survival (PFS).
Results
CT morphology was the independent LVI predictor. SUVmax provided better discrimination capability compared with CT morphology in the training set (P < 0.001) and test set (P = 0.042). A total of 1409 CT and PET radiomics features were extracted and reduced to 8, 8, and 10 features to build the 2D CT-RS, 3D CT-RS, and the PET-RS, respectively. There was no significant difference in AUC between the 2D-RS and 3D-RS (P > 0.05), and 2D CT-RS showed a relatively higher AUC than 3D CT-RS. The CT-RS, the CT-RNWOS, and the CT-RNWS showed good discrimination in the training set (AUC [area under the curve], 0.799, 0.796, and 0.851, respectively) and the test set (AUC, 0.818, 0.822, and 0.838, respectively). There was significant difference in AUC between the CT-RNWS and CT-RNWOS (P = 0.044) in the training set. Decision curve analysis (DCA) demonstrated the CT-RNWS outperformed the CT-RS and the CT-RNWOS in terms of clinical usefulness. Furthermore, DCA showed the PETCT-RNWS provided the highest net benefit compared with the PET-RNWS and CT-RNWS. PFS was significantly different between the pathologic and RNWS-predicted LVI-present and LVI-absent patients (P < 0.001). Carbohydrate antigen 125 (CA125), carcinoembryonic antigen (CEA), neuron-specific enolase (NSE), pathologic LVI, histologic subtype, and SUVmax were independent predictors of PFS in the 244 CT-RNWS-predicted cohort; and CA125, NSE, pathologic LVI, and SUVmax were the independent predictors of PFS in the 141 PETCT-RNWS-predicted cohort.
Conclusions
The radiomics nomogram, incorporating Rad-score, clinical and PET/CT parameters, shows favorable predictive efficacy for LVI status in LAC. Pathologic LVI and SUVmax are associated with LAC prognosis.





Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are not publicly available due to patient privacy concerns but are available from the corresponding author on reasonable request.
Change history
28 July 2020
Figures 1 and 4 are incorrect in the original manuscript.
References
Wang S, Zhang B, Qian J, Qiao R, Xu J, Zhang L, et al. Proposal on incorporating lymphovascular invasion as a T-descriptor for stage I lung cancer. Lung Cancer. 2018;125:245–52.
Sung SY, Kwak YK, Lee SW, Jo IY, Park JK, Kim KS, et al. Lymphovascular invasion increases the risk of nodal and distant recurrence in node-negative stage I-IIA non-small-cell lung cancer. Oncology. 2018;95:156–62.
Kinoshita T, Ohtsuka T, Yotsukura M, Asakura K, Goto T, Kamiyama I, et al. Prognostic impact of preoperative tumor marker levels and lymphovascular invasion in pathological stage I adenocarcinoma and squamous cell carcinoma of the lung. J Thorac Oncol. 2015;10:619–28.
Kuo SW, Chen JS, Huang PM, Hsu HH, Lai HS, Lee JM. Prognostic significance of histologic differentiation, carcinoembryonic antigen value, and lymphovascular invasion in stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2014;148:1200–7 e3.
Patel AJ, Daniel G, Naidu B, Bishay E. The significance of microvascular invasion after complete resection of early-stage non-small-cell lung cancer. Interact Cardiovasc Thorac Surg. 2016;22:101–5.
Okiror L, Harling L, Toufektzian L, King J, Routledge T, Harrison-Phipps K, et al. Prognostic factors including lymphovascular invasion on survival for resected non-small cell lung cancer. J Thorac Cardiovasc Surg. 2018;156:785–93.
Ramnefjell M, Aamelfot C, Aziz S, Helgeland L, Akslen LA. Microvascular proliferation is associated with aggressive tumour features and reduced survival in lung adenocarcinoma. J Pathol Clin Res. 2017;3:249–57.
Park C, Lee IJ, Jang SH, Lee JW. Factors affecting tumor recurrence after curative surgery for NSCLC: impacts of lymphovascular invasion on early tumor recurrence. J Thorac Dis. 2014;6:1420–8.
Mollberg NM, Bennette C, Howell E, Backhus L, Devine B, Ferguson MK. Lymphovascular invasion as a prognostic indicator in stage I non-small cell lung cancer: a systematic review and meta-analysis. Ann Thorac Surg. 2014;97:965–71.
Al-Alao BS, Gately K, Nicholson S, McGovern E, Young VK, O'Byrne KJ. Prognostic impact of vascular and lymphovascular invasion in early lung cancer. Asian Cardiovasc Thorac Ann. 2014;22:55–64.
Hishida T, Yoshida J, Maeda R, Ishii G, Aokage K, Nishimura M, et al. Prognostic impact of intratumoural microvascular invasion and microlymphatic permeation on node-negative non-small-cell lung cancer: which indicator is the stronger prognostic factor? Eur J Cardiothorac Surg. 2013;43:772–7.
Hanagiri T, Takenaka M, Oka S, Shigematsu Y, Nagata Y, Shimokawa H, et al. Prognostic significance of lymphovascular invasion for patients with stage I non-small cell lung cancer. Eur Surg Res. 2011;47:211–7.
Noma D, Inamura K, Matsuura Y, Hirata Y, Nakajima T, Yamazaki H, et al. Prognostic effect of lymphovascular invasion on TNM staging in stage I non-small-cell lung cancer. Clin Lung Cancer. 2018;19:e109–22.
Noda Y, Goshima S, Kanematsu M, Watanabe H, Kawada H, Kawai N, et al. F-18 FDG uptake on positron emission tomography as a predictor for lymphovascular invasion in patients with lung adenocarcinoma. Ann Nucl Med. 2016;30:11–7.
Tsuchiya N, Doai M, Usuda K, Uramoto H, Tonami H. Non-small cell lung cancer: whole-lesion histogram analysis of the apparent diffusion coefficient for assessment of tumor grade, lymphovascular invasion and pleural invasion. PLoS One. 2017;12:e0172433.
Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology. 2016;278:563–77.
Hassani C, Varghese BA, Nieva J, Duddalwar V. Radiomics in pulmonary lesion imaging. AJR Am J Roentgenol. 2019;212:497–504.
Thawani R, McLane M, Beig N, Ghose S, Prasanna P, Velcheti V, et al. Radiomics and radiogenomics in lung cancer: a review for the clinician. Lung Cancer. 2018;115:34–41.
Sollini M, Antunovic L, Chiti A, Kirienko M. Towards clinical application of image mining: a systematic review on artificial intelligence and radiomics. Eur J Nucl Med Mol Imaging. 2019. https://doi.org/10.1007/s00259-019-04372-x.
Kirienko M, Cozzi L, Rossi A, Voulaz E, Antunovic L, Fogliata A, et al. Ability of FDG PET and CT radiomics features to differentiate between primary and metastatic lung lesions. Eur J Nucl Med Mol Imaging. 2018;45:1649–60.
Yang X, He J, Wang J, Li W, Liu C, Gao D, et al. CT-based radiomics signature for differentiating solitary granulomatous nodules from solid lung adenocarcinoma. Lung Cancer. 2018;125:109–14.
Jiang M, Sun D, Guo Y, Guo Y, Xiao J, Wang L, et al. Assessing PD-L1 expression level by radiomic features from PET/CT in nonsmall cell lung cancer patients: an initial result. Acad Radiol. 2019. https://doi.org/10.1016/j.acra.2019.04.016.
Kang F, Mu W, Gong J, Wang S, Li G, Li G, et al. Integrating manual diagnosis into radiomics for reducing the false positive rate of (18)F-FDG PET/CT diagnosis in patients with suspected lung cancer. Eur J Nucl Med Mol Imaging. 2019. https://doi.org/10.1007/s00259-019-04418-0.
Tu W, Sun G, Fan L, Wang Y, Xia Y, Guan Y, et al. Radiomics signature: a potential and incremental predictor for EGFR mutation status in NSCLC patients, comparison with CT morphology. Lung Cancer. 2019;132:28–35.
Yu L, Tao G, Zhu L, Wang G, Li Z, Ye J, et al. Prediction of pathologic stage in non-small cell lung cancer using machine learning algorithm based on CT image feature analysis. BMC Cancer. 2019;19:464.
Ahn HK, Lee H, Kim SG, Hyun SH. Pre-treatment (18)F-FDG PET-based radiomics predict survival in resected non-small cell lung cancer. Clin Radiol. 2019;74:467–73.
Kirienko M, Cozzi L, Antunovic L, Lozza L, Fogliata A, Voulaz E, et al. Prediction of disease-free survival by the PET/CT radiomic signature in non-small cell lung cancer patients undergoing surgery. Eur J Nucl Med Mol Imaging. 2018;45:207–17.
Wang L, Dong T, Xin B, Xu C, Guo M, Zhang H, et al. Integrative nomogram of CT imaging, clinical, and hematological features for survival prediction of patients with locally advanced non-small cell lung cancer. Eur Radiol. 2019;29:2958–67.
Huang Y, Liu Z, He L, Chen X, Pan D, Ma Z, et al. Radiomics signature: a potential biomarker for the prediction of disease-free survival in early-stage (I or II) non-small cell lung cancer. Radiology. 2016;281:947–57.
Xu Y, Hosny A, Zeleznik R, Parmar C, Coroller T, Franco I, et al. Deep learning predicts lung cancer treatment response from serial medical imaging. Clin Cancer Res. 2019;25:3266–75.
Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ. 2015;350:g7594.
Nie P, Yang G, Wang Z, Yan L, Miao W, Hao D, et al. A CT-based radiomics nomogram for differentiation of renal angiomyolipoma without visible fat from homogeneous clear cell renal cell carcinoma. Eur Radiol. 2020;30:1274–84.
Liu J, Sun D, Chen L, Fang Z, Song W, Guo D, et al. Radiomics analysis of dynamic contrast-enhanced magnetic resonance imaging for the prediction of sentinel lymph node metastasis in breast cancer. Front Oncol. 2019;9:980.
Hyun SH, Eo JS, Song BI, Lee JW, Na SJ, Hong IK, et al. Preoperative prediction of microvascular invasion of hepatocellular carcinoma using (18)F-FDG PET/CT: a multicenter retrospective cohort study. Eur J Nucl Med Mol Imaging. 2018;45:720–6.
Xu X, Zhang HL, Liu QP, Sun SW, Zhang J, Zhu FP, et al. Radiomic analysis of contrast-enhanced CT predicts microvascular invasion and outcome in hepatocellular carcinoma. J Hepatol. 2019;70:1133–44.
Liu Z, Feng B, Li C, Chen Y, Chen Q, Li X, et al. Preoperative prediction of lymphovascular invasion in invasive breast cancer with dynamic contrast-enhanced-MRI-based radiomics. J Magn Reson Imaging. 2019. https://doi.org/10.1002/jmri.26688.
Liu S, Liu S, Ji C, Zheng H, Pan X, Zhang Y, et al. Application of CT texture analysis in predicting histopathological characteristics of gastric cancers. Eur Radiol. 2017;27:4951–9.
Shen C, Liu Z, Guan M, Song J, Lian Y, Wang S, et al. 2D and 3D CT radiomics features prognostic performance comparison in non-small cell lung cancer. Transl Oncol. 2017;10:886–94.
Shiono S, Abiko M, Sato T. Positron emission tomography/computed tomography and lymphovascular invasion predict recurrence in stage I lung cancers. J Thorac Oncol. 2011;6:43–7.
Funding
This study was funded by the National Natural Science Foundation of China (81701688 and 81601527); the Science and Technology Project of Southern District of Qingdao City (2020-2-004-YY); the Natural Science Foundation of Shandong Province (ZR2017BH096 and ZR2017MH036); the Key Research and Development Project of Shandong Province (2018GSF118078); and the Postdoctoral Science Foundation of China (2018 M642617).
Author information
Authors and Affiliations
Contributions
Literature search: Pei Nie, Guangjie Yang; Study design: Zhenguang Wang; Data collection: Pei Nie, Guangjie Yang, Yan Lei, Wenjie Miao, Yanli Duan, Yanli Wang, Aidi Gong, Yujun Zhao, Jie Wu, Chuantao Zhang, Maolong Wang, Mingming Yu, Dacheng Li; Data analysis: Guangjie Yang, Pei Nie, Ning Wang, Jingjing Cui, Yanqin Sun, Yangyang Wang; Manuscript writing: Pei Nie, Guangjie Yang; Manuscript review: Zhenguang Wang. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Research involving human participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee of The Affiliated Hospital of Qingdao University (Approval No. QYFY WZLL 25580) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was waived for this retrospective study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised. Figures 1 and 4 are updated.
This article is part of the Topical Collection on Oncology - Chest
Electronic supplementary material
ESM 1
(PDF 421 kb)
Rights and permissions
About this article
Cite this article
Nie, P., Yang, G., Wang, N. et al. Additional value of metabolic parameters to PET/CT-based radiomics nomogram in predicting lymphovascular invasion and outcome in lung adenocarcinoma. Eur J Nucl Med Mol Imaging 48, 217–230 (2021). https://doi.org/10.1007/s00259-020-04747-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-020-04747-5