Abstract
Purpose
To evaluate whether standardized uptake value (SUV) and/or metabolic rate of glucose (MRglu) are different among epithelioid, mixed, and spindle cell uveal melanomas, as well as between low and high risk melanomas; to correlate ultrasonographic data and metabolic parameters with histopathological features; and to assess the role of 18F-FDG PET/CT for evaluating prognosis.
Methods
Of 34 eligible patients prospectively enrolled with clinical suspicion of medium/large uveal melanoma, 26 (15 men, mean age 62.8 ± 11.8 years) were evaluated. All patients underwent metastatic work-up, 3-D dynamic brain and whole-body 18F-FDG PET/CT, and surgery.
Results
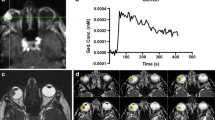
Of the 26 ocular lesions, 23 showed 18F-FDG uptake, with a sensitivity of 88 %. MRglu was significantly higher in the epithelioid cell melanomas than in the spindle cell melanomas, as well as in high-risk lesions than in low-risk lesions (p = 0.01, p = 0.02, respectively). SUV and MRglu were correlated with histopathological features while ultrasonographic data were not.
Conclusion
MRglu is useful for distinguishing the different cell types in uveal melanoma, as well as high-risk from low-risk lesions, while SUV is not. MRglu provides a more accurate evaluation of glucose consumption, whereas SUV provides only an estimation. In addition, the metabolic parameters correlate with histopathological features, well also reflecting cellular behaviour in ocular malignancy. A longer follow-up is needed to assess the role of 18F-FDG in evaluating prognosis.



Similar content being viewed by others
References
Virgili G, Gatta G, Ciccolallo L, Capocaccia R, Biggeri A, Crocetti E, et al. Incidence of uveal melanoma in Europe. Ophthalmology. 2007;114:2309–15.
Ambati J, Anand A, Fernandez S, Sakurai E, Lynn BC, Kuziel WA, et al. Baseline echographic characteristics of tumors in eyes of patients enrolled in the Collaborative Ocular Melanoma Study: COMS report no. 29. Ophthalmology. 2008;115:1390–7.
Shildkrot Y, Wilson MW. Update on posterior uveal melanoma: treatment of the eye and emerging strategies in the prognosis and treatment of metastatic disease. Curr Opin Ophthalmol. 2009;20:504–10.
Damato B. Progress in the management of patients with uveal melanoma. The 2012 Ashton Lecture. Eye. 2012;26:1157–72.
Patel M, Smyth E, Chapman PB, Wolchok JD, Schwartz GK, Abramson DH, et al. Therapeutic implications of the emerging molecular biology of uveal melanoma. Clin Cancer Res. 2011;17:2087–100.
Edge SB. Malignant melanoma of the uvea. In: Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7th ed. New York: Springer; 2010. p. 547–53.
Finger PT, Chin K, Iacob CE. 18-Fluorine-labelled 2-deoxy-2-fluoro-D-glucose positron emission tomography/computed tomography standardised uptake values: a non-invasive biomarker for the risk of metastasis from choroidal melanoma. Br J Ophthalmol. 2006;90:1263–6.
Loercher AE, Harbour JW. Molecular genetics of uveal melanoma. Curr Eye Res. 2003;27:69–74.
McCannel TA, Reddy S, Burgess BL, Auerbach M. Association of positive dual-modality positron emission tomography/computed tomography imaging of primary choroidal melanoma with chromosome 3 loss and tumor size. Retina. 2010;30:146–51.
Finger PT, Kurli M, Reddy S, Tena LB, Pavlick AC. Whole body PET/CT for initial staging of choroidal melanoma. Br J Ophthalmol. 2005;89:1270–4.
Lucignani G, Paganelli G, Modorati G, Pieralli S, Rizzo G, Magnani P, et al. MRI, antibody-guided scintigraphy, and glucose metabolism in uveal melanoma. J Comput Assist Tomogr. 1992;16:77–83.
Spraul CW, Lang GE, Lang GK. Value of positron emission tomography in the diagnosis of malignant ocular tumors. Ophthalmologica. 2001;215:163–8.
Modorati G, Lucignani G, Landoni C, Freschi M, Trabucchi G, Fazio F, et al. Glucose metabolism and pathological findings in uveal melanoma: preliminary results. Nucl Med Commun. 1996;17:1052–6.
Reddy S, Kurli M, Tena LB, Finger PT. PET/CT imaging: detection of choroidal melanoma. Br J Ophthalmol. 2005;89:1265–9.
Singh AD, Bhatnagar P, Bybel B. Visualization of primary uveal melanoma with PET/CT scan. Eye. 2006;20:938–40.
Faia LJ, Pulido JS, Donaldson MJ, Diva RS, Cameron JD, Mullan B, et al. The relationship between combined positron emission tomography/computed tomography and clinical and light microscopic findings in choroidal melanoma. Retina. 2008;28:763–9.
Freton A, Chin KJ, Raut R, Tena LB, Kivela T, Finger PT. Initial PET/CT staging for choroidal melanoma: AJCC correlation and second nonocular primaries in 333 patients. Eur J Ophthalmol. 2010;22:236–43.
Finger PT, Chin KJ. [18F]Fluorodeoxyglucose positron emission tomography/computed tomography (PET/CT) physiologic imaging of choroidal melanoma: before and after ophthalmic plaque radiation therapy. Int J Radiat Oncol Biol Phys. 2011;79:137–42.
Lee CS, Cho A, Lee KS, Lee SC. Association of high metabolic activity measured by positron emission tomography imaging with poor prognosis of choroidal melanoma. Br J Ophthalmol. 2011;95:1588–91.
Kato K, Kubota T, Ikeda M, Tadokoro M, Abe S, Nakano S, et al. Low efficacy of 18F-FDG PET for detection of uveal malignant melanoma compared with 123I-IMP SPECT. J Nucl Med. 2006;47:404–9.
Yamada K, Brink I, Bissé E, Epting T, Engelhardt R. Factors influencing [F-18] 2-fluoro-2-deoxy-D-glucose (F-18 FDG) uptake in melanoma cells: the role of proliferation rate, viability, glucose transporter expression and hexokinase activity. J Dermatol. 2005;32:316–34.
Basu S, Zaidi H, Alavi A. Clinical and research applications of quantitative PET imaging. PET Clin. 2007;2:161–72.
Nag S, Quivey JM, Earle JD, Followill D, Fontanesi J, Finger PT. The American Brachytherapy Society reccomendations for brachytherapy of uveal melanomas. Int J Radiat Oncol Biol Phys. 2003;56:544–55.
Burger C, Buck A. Requirements and implementation of a flexible kinetic modelling tool. J Nucl Med. 1997;38:1818–23.
Reivich M, Alavi A, Wolf A, Fowler J, Russell J, Arnett C, et al. Glucose metabolic rate kinetic model parameter determination in humans: the lumped constants and rate constants for [18F]fluorodeoxyglucose and [11C]deoxyglucose. J Cereb Blood Flow Metab. 1985;5:179–92.
Chen K, Bandy D, Reiman E, Huang SC, Lawson M, Feng D, et al. Noninvasive quantification of the cerebral metabolic rate for glucose using positron emission tomography, 18F-fluoro-2-deoxyglucose, the Patlak method, and an image-derived input function. J Cereb Blood Flow Metab. 1998;18:716–23.
Kim CK, Gupta NC, Chandramouli B, Alavi A. Standardized uptake values of FDG: body surface area correction is preferable to body weight correction. J Nucl Med. 1994;35:164–7.
Srinivas SM, Dhurairaj T, Basu S, Bural G, Surti S, Alavi A. A recovery coefficient method for partial volume correction of PET images. Ann Nucl Med. 2009;23:341–8.
Scupola A, Blasi MA, Petroni S, Tiberti AC, Mastrocola A, Caputo CG, et al. Bovine pericardium for scleral closure in transscleral local resection of choroidal melanoma. Retina. 2008;28:1530–2.
McLean IW, Foster WD, Zimmerman LE, Gamel JW. Modifications of Callender’s classification of uveal melanoma at the Armed Forces Institute of Pathology. Am J Ophthalmol. 1983;96:502–9.
Callender GR. Malignant melanoma tumors of eye: study of histologic types in Ill cases. Trans Am Acad Ophthalmol Otolaringol. 1931;36:131–42.
Foss AJ, Alexander RA, Hungerford JL, Harris AL, Cree IA, Lightman S. Reassessment of the PAS patterns in uveal melanoma. Br J Ophthalmol. 1997;81:240–8.
Khalaf M, Abdel-Nabi H, Baker J, Shao Y, Lamonica D, Gona J. Relation between nodule size and 18F-FDG-PET SUV for malignant and benign pulmonary nodules. J Hematol Oncol. 2008;1:13.
Heyneman LF, Patz EF. PET imaging in patients with bronchioloalveolar cell carcinoma. Lung Cancer. 2002;38:261–6.
Daniels CE, Lowe VJ, Aubry MC, Allen MS, Jett JR. The utility of fluorodeoxyglucose positron emission tomography in the evaluation of carcinoid tumors presenting as pulmonary nodules. Chest. 2007;131:255–60.
Al-Jamal RT, Kivelä T. KI-67 immunopositivity in choroidal and ciliary body melanoma with respect to nucleolar diameter and other prognostic factors. Curr Eye Res. 2006;3:57–67.
Mouriaux F, Diorio C, Bergeron D, Berchi C, Rousseau. Liver function testing is not helpful for early diagnosis of metastatic uveal melanoma. Ophthalmology. 2012;119:1590–5.
Shields CL, Furuta M, Thangappan A, Nagori S, Mashayekhi A, Lally DR, et al. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch Ophthalmol. 2009;127:989–98.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Calcagni, M.L., Mattoli, M.V., Blasi, M.A. et al. A prospective analysis of 18F-FDG PET/CT in patients with uveal melanoma: comparison between metabolic rate of glucose (MRglu) and standardized uptake value (SUV) and correlations with histopathological features. Eur J Nucl Med Mol Imaging 40, 1682–1691 (2013). https://doi.org/10.1007/s00259-013-2488-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-013-2488-6