Abstract
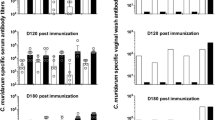
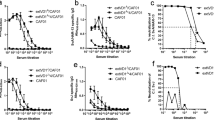
Chlamydia trachomatis is the leading cause of sexually transmitted infections worldwide. There is currently no commercially available vaccine against C. trachomatis. Major outer membrane protein (MOMP) of C. trachomatis is considered to be an ideal candidate for prophylactic vaccine. We designed a MOMP multi-epitope containing T- and B-cell epitope-rich peptides and developed hepatitis B surface antigen (HBsAg) as antigen delivery vehicle. In order to study the immunogenicity and efficacy of the candidate vaccine in a murine model of chlamydial genital infection, we engineered a recombinant plasmid expressing HBsAg and MOMP multi-epitope genes. Results of reverse transcription polymerase chain reaction and immunofluorescence assay revealed successful expression of the recombinant HBsAg/MOMP multi-epitope gene at both the transcription and translation levels. Intramuscular administration in mice was able to elicit not only antibodies against Chlamydia and HBsAg but also cytotoxic T lymphocyte activity against Chlamydia. In addition, mice inoculated with the rHBsAg were highly resistant to C. trachomatis genital infection. The rHBsAg DNA with MOMP multi-epitope appended at the C terminus of the HBsAg stimulated a stronger immune response and protective response than that appended at the N terminus. Together, our results suggested that use of a recombinant HBsAg encoding the MOMP multi-epitope could be a powerful approach to developing a safe and immunogenic C. trachomatis vaccine.







Similar content being viewed by others
References
Andersen B, Ostergaard L, Puho E, Skriver MV, Schonheyder HC (2005) Ectopic pregnancies and reproductive capacity after Chlamydia trachomatis positive and negative test results: a historical follow-up study. Sex Transm Dis 32(6):377–381
Arya R, Mannion PT, Woodcock K, Haddad NG (2005) Incidence of genital Chlamydia trachomatis infection in the male partners attending an infertility clinic. J Obstet Gynaecol 25(4):364–367
Báez-Astúa A, Herráez-Hernández E, Garbi N, Pasolli HA, Juárez V, Zur Hausen H, Cid-Arregui A (2005) Low-dose adenovirus vaccine encoding chimeric hepatitis B virus surface antigen-human papillomavirus type 16 E7 proteins induces enhanced E7-specific antibody and cytotoxic T-cell responses. J Virol 79(20):12807–12817
Bax CJ, Quint KD, Peters RP, Ouburg S, Oostvogel PM, Mutsaers JA, Dörr PJ, Schmidt S, Jansen C, van Leeuwen AP, Quint WG, Trimbos JB, Meijer CJ, Morré SA (2011) Analyses of multiple-site and concurrent Chlamydia trachomatis serovar infections, and serovar tissue tropism for urogenital versus rectal specimens in male and female patients. Sex Transm Infect 87(6):503–507
Berkower I, Raymond M, Muller J, Spadaccini A, Aberdeen A (2004) Assembly, structure, and antigenic properties of virus-like particles rich in HIV-1 envelope gp120. Virology 321(1):75–86
Berry LJ, Hickey DK, Skelding KA, Bao S, Rendina AM, Hansbro PM, Gockel CM, Beagley KW (2004) Transcutaneous immunization with combined cholera toxin and CpG adjuvant protects against Chlamydia muridarum genital tract infection. Infect Immun 72(2):1019–1028
Chen L, Lei L, Chang X, Li Z, Lu C, Zhang X, Wu Y, Yeh IT, Zhong G (2010) Mice deficient in MyD88 develop a Th2-dominant response and severe pathology in the upper genital tract following Chlamydia muridarum infection. J Immunol 184(5):2602–2610
Cheong WS, Reiseger J, Turner SJ, Boyd R, Netter HJ (2009) Chimeric virus-like particles for the delivery of an inserted conserved influenza A-specific CTL epitope. Antiviral Res 81(2):113–122
de Barbeyrac B (2013) Current aspects of Chlamydia trachomatis infection. Presse Med 42(4 Pt 1):440–445
de la Maza LM, Peterson EM (2002) Vaccines for Chlamydia trachomatis infections. Curr Opin Investig Drugs 3:980–986
Delpeyroux F, Van Wezel E, Blondel B, Crainic R (1990) Structural factors modulate the activity of antigenic polio-virus sequences expressed on hybrid hepatitis B surface antigen particles. J Virol 64:6090–6100
Donati M, Di Francesco A, D’Antuono A, Pignanelli S, Shurdhi A, Moroni A, Baldelli R, Cevenini R (2009) Chlamydia trachomatis serovar distribution and other concurrent sexually transmitted infections in heterosexual men with urethritis in Italy. Eur J Clin Microbiol Infect Dis 28:523–526
Eko FO, He Q, Brown T, McMillan L, Ifere GO, Ananaba GA, Lyn D, Lubitz W, Kellar KL, Black CM, Igietseme JU (2004) A novel recombinant multisubunit vaccine against Chlamydia. J Immunol 173(5):3375–3382
Eko FO, Barisani-Asenbauer T, Lubitz W (2008) Development of a Chlamydia trachomatis bacterial ghost vaccine to fight human blindness. Hum Vaccine 4(3):176–183
Ercolini AM, Machiels JP, Chen YC, Slansky JE, Giedlen M, Reilly RT, Jaffee EM (2003) Identification and characterization of the immunodominant rat HER/neu MHC class I epitope presented by spontaneous mammary tumors from HER-2/neu-transgenic mice. J Immunol 170(8):4273–4280
Gonzales GF, Muñoz G, Sánchez R, Henkel R, Gallegos-Avila G, Díaz-Gutierrez O, Vigil P, Vásquez F, Kortebani G, Mazzolli A, Bustos-Obregón E (2004) Update on the impact of Chlamydia trachomatis infection on male fertility. Andrologia 36(1):1–23
Greene W, Xiao Y, Huang Y, McClarty G, Zhong G (2004) Chlamydia-infected cells continue to undergo mitosis and resist induction of apoptosis. Infect Immun 72(1):451–460
Hafner LM, McNeilly C (2008) Vaccines for Chlamydia infections of the female genital tract. Future Microbiol 3:67–77
Haigh O, Kattenbelt J, Cochrane M, Thomson S, Gould A, Tindle R (2010) Hepatitis B surface antigen fusions delivered by DNA vaccination elicit CTL responses to human papillomavirus oncoproteins associated with tumor protection. Cancer Gene Ther 17(10):708–720
Holland MJ, Conway DJ, Blanchard TJ, Mahdi OM, Bailey RL, Whittle HC, Mabey DC (1997) Synthetic peptides based on Chlamydia trachomatis antigens identify cytotoxic T lymphocyte responses in subjects from a trachoma-endemic population. Clin Exp Immunol 107(1):44–49
Igietseme JU, Murdin A (2000) Induction of protective immunity against Chlamydia trachomatis genital infection by a vaccine based on major outer membrane protein-lipophilic immune response-stimulating complexes. Infect Immun 68(12):6798–6806
Kamel RM (2013) Screening for Chlamydia trachomatis infection among infertile women in Saudi Arabia. Int J Womens Health 5:277–284
Kari L, Whitmire WM, Crane DD, Reveneau N, Carlson JH, Goheen MM, Peterson EM, Pal S, de la Maza LM, Caldwell HD (2009) Chlamydia trachomatis native major outer membrane protein induces partial protection in nonhuman primates: implication for a trachoma transmission blocking vaccine. J Immunol 182(12):8063–8070
Komoda T (2007) Kinetic study of antibodies (IgG, IgA) to Chlamydia trachomatis: importance of IgA antibody in screening test for C. trachomatis infection by peptide-based enzyme immunosorbent assay. Jpn J Infect Dis 60(6):347–351
Kotiw M, Johnson M, Pandey M, Fry S, Hazell SL, Netter HJ, Good MF, Olive C (2012) Immunological response to parenteral vaccination with hepatitis B virus surface antigen virus-like particles expressing Helicobacter pylori KatA epitopes in a murine H. pylori challenge model. Clin Vaccine Immunol 19(2):268–276
Kubo A, Stephens RS (2000) Characterization and functional analysis of PorB, a Chlamydia porin and neutralizing target. Mol Microbiol 38:772–780
Lee IH, Kim CH, Ryu WS (1996) Presentation of the hydrophilic domains of hepatitis C viral E2 envelope glycoprotein on hepatitis B surface antigen particles. J Med Virol 50:145–151
Longbottom D (2003) Chlamydial vaccine development. J Med Microbiol 52(Pt 7):537–540
Lu Y, Xiao Y, Ding J, Dierich M, Chen YH (2000) Immunogenicity of neutralizing epitopes on multiple-epitope vaccines against HIV-1. Int Arch Allergy Immunol 121(1):80–84
Lu H, Xing Z, Brunham RC (2002) GM-CSF transgene-based adjuvant allows the establishment of protective mucosal immunity following vaccination with inactivated Chlamydia trachomatis. J Immunol 169(11):6324–6331
Marsac D, Puaux AL, Rivière Y, Michel ML (2005) In vivo induction of cellular and humoral immune responses by hybrid DNA vectors encoding simian/human immunodeficiency virus/hepatitis B surface antigen virus particles in BALB/c and HLA-A2-transgenic mice. Immunobiology 210(5):305–319
Michel M, Lone YC, Centlivre M, Roux P, Wain-Hobson S, Sala M (2007) Optimisation of secretion of recombinant HBsAg virus-like particles: impact on the development of HIV-1/HBV bivalent vaccines. Vaccine 25(10):1901–1911
Netter HJ, Macnaughton TB, Woo WP, Tindle R, Gowans EJ (2001) Antigenicity and immunogenicity of novel chimeric hepatitis B surface antigen particles with exposed hepatitis C virus epitopes. J Virol 75(5):2130–2141
Ortiz L, Demick KP, Petersen JW, Polka M, Rudersdorf RA, Van der Pol B, Jones R, Angevine M, DeMars R (1996) Chlamydia trachomatis major outer membrane protein (MOMP) epitopes that activate HLA class II-restricted T cells from infected humans. J Immunol 157(10):4554–4567
Petrovay F, Balla E, Ne’meth I, Go¨nczo¨ l E (2009) Genotyping of Chlamydia trachomatis from the endocervical specimens of high-risk women in Hungary. J Med Microbiol 58:760–764
Phogat S, Svehla K, Tang M, Spadaccini A, Muller J, Mascola J, Berkower I, Wyatt R (2008) Analysis of the human immunodeficiency virus type 1 gp41 membrane proximal external region arrayed on hepatitis B surface antigen particles. Virology 373(1):72–84
Qian B, Shen H, Liang W, Guo X, Zhang C, Wang Y, Li G, Wu A, Cao K, Zhang D (2008) Immunogenicity of recombinant hepatitis B virus surface antigen fused with preS1 epitopes expressed in rice seeds. Transgenic Res 17(4):621–631
Roy MJ, Wu MS, Barr LJ, Fuller JT, Tussey LG, Speller S, Culp J, Burkholder JK, Swain WF, Dixon RM, Widera G, Vessey R, King A, Ogg G, Gallimore A, Haynes JR, Heydenburg Fuller D (2000) Induction of antigen-specific CD8+ T cells, T helper cells, and protective levels of antibody in humans by particle mediated administration of a hepatitis B virus DNA vaccine. Vaccine 19:764–778
Schautteet K, De Clercq E, Vanrompay D (2011) Chlamydia trachomatis vaccine research through the years. Infect Dis Obstet Gynecol 2011:963513
Schirmbeck R, Reimann J (2002) Alternative processing of endogenous or exogenous antigens extends the immunogenic, H-2 class I-restricted peptide repertoire. Mol Immunol 39:249–259
Shah AA, Schripsema JH, Imtiaz MT, Sigar IM, Kasimos J, Matos PG, Inouye S, Ramsey KH (2005) Histopathologic changes related to fibrotic oviduct occlusion after genital tract infection of mice with Chlamydia muridarum. Sex Transm Dis 32(1):49–56
Stevenson FK, Ottensmeier CH, Johnson P, Zhu D, Buchan SL, McCann KJ, Roddick JS, King AT, McNicholl F, Savelyeva N, Rice J (2004) DNA vaccines to attack cancer. Proc Natl Acad Sci U S A 101(Suppl 2):14646–14652
Stothard DR, Boguslawski G, Jones RB (1998) Phylogenetic analysis of the Chlamydia trachomatis major outer membrane protein and examination of potential pathogenic determinants. Infect Immun 66:3618–3625
Sun G, Pal S, Weiland J, Peterson EM, de la Maza LM (2009) Protection against an intranasal challenge by vaccines formulated with native and recombinant preparations of the Chlamydia trachomatis major outer membrane protein. Vaccine 27:5020–5025
Swanson KA, Taylor LD, Frank SD, Sturdevant GL, Fischer ER, Carlson JH, Whitmire WM, Caldwell HD (2009) Chlamydia trachomatis polymorphic membrane protein D is an oligomeric autotransporter with a higher-order structure. Infect Immun 77:508–516
Tang L, Zhang H, Lei L, Gong S, Zhou Z, Baseman J, Zhong G (2013) Oviduct infection and hydrosalpinx in DBA1/j mice is induced by intracervical but not intravaginal inoculation with Chlamydia muridarum. PLoS One 8(8):e71649
Vietheer PT, Boo I, Drummer HE, Netter HJ (2007) Immunizations with chimeric hepatitis B virus-like particles to induce potential anti-hepatitis C virus neutralizing antibodies. Antivir Ther 12(4):477–487
Xu W, Liu J, Gong W, Chen J, Zhu S, Zhang L (2011) Protective immunity against Chlamydia trachomatis genital infection induced by a vaccine based on the major outer membrane multi-epitope human papillomavirus major capsid protein L1. Vaccine 29(15):2672–2678
Zhu SL, Shi ZH, Li WS, Chen J, Zhang LF (2009) Immunogenicity of multi- epitopes gene of major outer membrane protein of Chlamydia trachomatis. Zhonghua Yu Fang Yi Xue Za Zhi 43(3):232–236
Acknowledgments
This work was supported by the grant from the National Natural Science Foundation of China (no. 30972669), Zhejiang Provincial Natural Science Foundation of China (nos. Y2100909, Y2100660, and Y2100611), and Foundation of Science and Technology Bureau of Wenzhou City (no. Y20100090).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 77 kb)
Rights and permissions
About this article
Cite this article
Zhu, S., Feng, Y., Rao, P. et al. Hepatitis B virus surface antigen as delivery vector can enhance Chlamydia trachomatis MOMP multi-epitope immune response in mice. Appl Microbiol Biotechnol 98, 4107–4117 (2014). https://doi.org/10.1007/s00253-014-5517-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5517-x