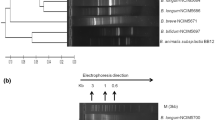
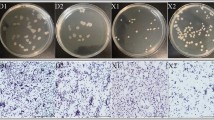
Abstract
Bacillus spp. are commonly used as probiotic species in the feed industry, however, their benefits need to be confirmed. This study describes a high throughput screening combined with the detailed characterization of endospore-forming bacteria with the aim to identify new Bacillus spp. strains for use as probiotic additives in pig feed. A total of 245 bacterial isolates derived from African fermented food, feces and soil were identified by 16S rRNA gene sequencing and screened for antimicrobial activity and growth in the presence of antibiotics, bile salts and at pH 4.0. Thirty-three Bacillus spp. isolates with the best characteristics were identified by gyrB and rpoB gene sequencing as B. amyloliquefaciens subsp. plantarum, B. amyloliquefaciens subsp. amyloliquefaciens, B. subtilis subsp. subtilis, B. licheniformis, B. mojavensis, B. pumilus and B. megaterium. These isolates were further investigated for their activity against the pathogenic bacteria, antibiotic susceptibility, sporulation rates, biofilm formation and production of glycosyl hydrolytic enzymes. Additionally, ten selected isolates were assessed for heat resistance of spores and the effect on porcine epithelial cells IPEC-J2. Isolates of B. amyloliquefaciens, B. subtilis and B. mojavensis, showed the best overall characteristics and, therefore, potential for usage as probiotic additives in feed. A large number of taxonomically diverse strains made it possible to reveal species and subspecies-specific trends, contributing to our understanding of the probiotic potential of Bacillus species.





Similar content being viewed by others
References
Abou-Taleb KAA, Mashhoor WA, Nasr SA, Sharaf MS, Abdel-Azeem HHM (2009) Nutritional and environmental factors affecting cellulase production by two strains of cellulolytic Bacilli. Australian. J Basic Applied Sci 3:2429–2436
Abriouel H, Franz CM, Ben ON, Galvez A (2011) Diversity and applications of Bacillus bacteriocins. FEMS Microbiol Rev 35:201–232. doi:10.1111/j.1574-6976.2010.00244.x
Adimpong DB, Sorensen KI, Thorsen L, Stuer-Lauridsen B, Abdelgadir WS, Nielsen DS, Derkx PM, Jespersen L (2012) Antimicrobial susceptibility, characterization of bacitracin operon and bacitracin biosynthesis of Bacillus spp. strains isolated from primary starters for African traditional bread production. Appl Environ Microbiol 78:7903–7914. doi:10.1128/AEM.00730-12
Alexopoulos C, Georgoulakis IE, Tzivara A, Kyriakis CS, Govaris A, Kyriakis SC (2004) Field evaluation of the effect of a probiotic-containing Bacillus licheniformis and Bacillus subtilis spores on the health status, performance, and carcass quality of grower and finisher pigs. J Vet Med A Physiol Pathol Clin Med 51:306–312. doi:10.1111/j.1439-0442.2004.00637
Auger S, Ramarao N, Faille C, Fouet A, Aymerich S, Gohar M (2009) Biofilm formation and cell surface properties among pathogenic and nonpathogenic strains of the Bacillus cereus group. Appl Environ Microbiol 75:6616–6618. doi:10.1128/AEM.00155-09
Barbosa TM, Serra CR, La Ragione RM, Woodward MJ, Henriques AO (2005) Screening for bacillus isolates in the broiler gastrointestinal tract. Appl Environ Microbiol 71:968–978. doi:10.1128/AEM.71.2.968-978.2005
Borriss R, Chen XH, Rueckert C, Blom J, Becker A, Baumgarth B, Fan B, Pukall R, Schumann P, Sproer C, Junge H, Vater J, Puhler A, Klenk HP (2011) Relationship of Bacillus amyloliquefaciens clades associated with strains DSM 7 T and FZB42T: a proposal for Bacillus amyloliquefaciens subsp. amyloliquefaciens subsp. nov. and Bacillus amyloliquefaciens subsp. plantarum subsp. nov. based on complete genome sequence comparisons. Int J Syst Evol Microbiol 61:1786–1801. doi:10.1099/ijs.0.023267-0
Branda SS, Gonzalez-Pastor JE, Ben-Yehuda S, Losick R, Kolter R (2001) Fruiting body formation by Bacillus subtilis. Proc Natl Acad Sci U S A 98:11621–11626. doi:10.1073/pnas.191384198
Clinical and Laboratory Standards Institute (2010) Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria; Approved guideline, 2nd Edition. CLSI document M45-A2 (ISBN 1-56238-732-4)
Compaore CS, Nielsen DS, Sawadogo-Lingani H, Berner TS, Nielsen FK, Adimpong DB, Diawara B, Ouedraogo GA, Jakobsen M, Thorsen L (2013) Bacillus amyloliquefaciens susbsp. plantarum strains as potential protective starter cultures for the production of bikalga, an alkaline fermented food. J. Appl. Microbiol Apr 9. doi: 10.1111/jam.12214. [Epub ahead of print]
Cordeiro CAM, Martins MLL, Luciano AB, da Silva RF (2002) Production and properties of xylanase from thermophilic Bacillus sp. Braz Arch Biol Technol 45:413–418. doi:10.1590/S1516-89132002000600002
Cutting SM (2011) Bacillus probiotics. Food Microbiol 28:214–220. doi:10.1016/j.fm.2010.03.007
Diaz D (2007) Effect of Bacillus amyloliquefaciens CECT-5940 spores on broiler performance and digestibility. Published online: http://en.engormix.com/MA-poultry-industry/articles/effect-bacillus-amyloliquefaciens-cect5940-t795/p0.htm
Dischinger J, Josten M, Szeka C, Sahl HG, Bierbaum G (2009) Production of the novel two-peptide lantibiotic lichenicidin by Bacillus licheniformis DSM 13. PLoS One 4:e6788. doi:10.1371/journal.pone.0006788
Duc LH, Hong HA, Barbosa TM, Henriques AO, Cutting SM (2004) Characterization of Bacillus probiotics available for human use. Appl Environ Microbiol 70:2161–2171. doi:10.1128/AEM.70.4.2161-2171.2004
EFSA (2008) Scientific Opinion of the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) on a request from the European Commission on the safety and efficacy of Ecobiol® (Bacillus amyloliquefaciens) as feed additive for chickens for fattening. EFSA Journal 2008 773: 1–13. http://www.efsa.europa.eu/en/scdocs/doc/773
EFSA (2010a) EFSA Panel on Biological Hazards (BIOHAZ); Scientific Opinion on the maintenance of the list of QPS biological agents intentionally added to food and feed. EFSA Journal 2010 8:1944. http://www.efsa.europa.eu/en/efsajournal/pub/1944.htm
EFSA (2010b) EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP); Scientific Opinion on the safety and efficacy of Calsporin® (Bacillus subtilis) as a feed additive for piglets on request from the European Commission. EFSA Journal 2010 8:1426. http://www.efsa.europa.eu/en/efsajournal/pub/1426.htm
EFSA (2011a) EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP); Scientific Opinion on the safety and efficacy of BioPlus 2B (Bacillus licheniformis DSM 5749 and Bacillus subtilis DSM 5750) as a feed additive for sows. EFSA Journal 2011 9:2356. http://www.efsa.europa.eu/en/efsajournal/pub/2356.htm
EFSA (2011b) EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP); Technical Guidance on the assessment of the toxigenic potential of Bacillus species used in animal nutrition. EFSA Journal 2011 9:2445. http://www.efsa.europa.eu/en/efsajournal/pub/2445.htm
EFSA (2012) EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP); Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA Journal 2012 10:2740. http://www.efsa.europa.eu/en/efsajournal/pub/2740.htm
Fakhry S, Sorrentini I, Ricca E, De FM, Baccigalupi L (2008) Characterization of spore forming Bacilli isolated from the human gastrointestinal tract. J Appl Microbiol 105:2178–2186. doi:10.1111/j.1365-2672.2008.03934
Gaggia F, Mattarelli P, Biavati B (2010) Probiotics and prebiotics in animal feeding for safe food production. Int J Food Microbiol 141:S15–S28. doi:10.1016/j.ijfoodmicro.2010.02.031
Guo X, Li D, Lu W, Piao X, Chen X (2006) Screening of Bacillus strains as potential probiotics and subsequent confirmation of the in vivo effectiveness of Bacillus subtilis MA139 in pigs. Antonie Van Leeuwenhoek 90:139–146. doi:10.1007/s10482-006-9067-9
Hoa NT, Baccigalupi L, Huxham A, Smertenko A, Van PH, Ammendola S, Ricca E, Cutting AS (2000) Characterization of Bacillus species used for oral bacteriotherapy and bacterioprophylaxis of gastrointestinal disorders. Appl Environ Microbiol 66:5241–5247. doi:10.1128/AEM.66.12.5241-5247.2000
Hong HA, Duc LH, Cutting SM (2005) The use of bacterial spore formers as probiotics. FEMS Microbiol Rev 29:813–835. doi:10.1016/j.femsre.2004.12.001
Hong HA, Khaneja R, Tam NM, Cazzato A, Tan S, Urdaci M, Brisson A, Gasbarrini A, Barnes I, Cutting SM (2009a) Bacillus subtilis isolated from the human gastrointestinal tract. Res Microbiol 160:134–143. doi:10.1016/j.resmic.2008.11.002
Hong HA, To E, Fakhry S, Baccigalupi L, Ricca E, Cutting SM (2009b) Defining the natural habitat of Bacillus spore-formers. Res Microbiol 160:375–379. doi:10.1016/j.resmic.2009.06.006
Hosoi T, Hirose R, Saegusa S, Ametani A, Kiuchi K, Kaminogawa S (2003) Cytokine responses of human intestinal epithelial-like Caco-2 cells to the nonpathogenic bacterium Bacillus subtilis (natto). Int J Food Microbiol 82:255–264. doi:10.1016/S0168-1605(02)00311-2
Huang CH, Chang MT, Huang L, Chu WS (2012) Development of a novel PCR assay based on the gyrase B gene for species identification of Bacillus licheniformis. Mol Cell Probes 26:215–217. doi:10.1016/j.mcp.2012.05.001
Kabore D, Thorsen L, Nielsen DS, Berner TS, Sawadogo-Lingani H, Diawara B, Dicko MH, Jakobsen M (2012) Bacteriocin formation by dominant aerobic sporeformers isolated from traditional maari. Int J Food Microbiol 154:10–18. doi:10.1016/j.ijfoodmicro.2011.12.003
Ki JS, Zhang W, Qian PY (2009) Discovery of marine Bacillus species by 16S rRNA and rpoB comparisons and their usefulness for species identification. J Microbiol Methods 77:48–57. doi:10.1016/j.mimet.2009.01.003
Kort R, O'Brien AC, van Stokkum IH, Oomes SJ, Crielaard W, Hellingwerf KJ, Brul S (2005) Assessment of heat resistance of bacterial spores from food product isolates by fluorescence monitoring of dipicolinic acid release. Appl Environ Microbiol 71:3556–3564. doi:10.1128/AEM.71.7.3556-3564.2005
La Ragione RM, Woodward MJ (2003) Competitive exclusion by Bacillus subtilis spores of Salmonella enterica serotype Enteritidis and Clostridium perfringens in young chickens. Vet Microbiol 94:245–256. doi:10.1016/S0378-1135(03)00077-4
Leser TD, Knarreborg A, Worm J (2008) Germination and outgrowth of Bacillus subtilis and Bacillus licheniformis spores in the gastrointestinal tract of pigs. J Appl Microbiol 104:1025–1033. doi:10.1111/j.1365-2672.2007.03633.x
Ludwig W, Strunk O, Westram R, Richter L, Meier H, Yadhukumar Buchner A, Lai T, Steppi S, Jobb G, Forster W, Brettske I, Gerber S, Ginhart AW, Gross O, Grumann S, Hermann S, Jost R, Konig A, Liss T, Lussmann R, May M, Nonhoff B, Reichel B, Strehlow R, Stamatakis A, Stuckmann N, Vilbig A, Lenke M, Ludwig T, Bode A, Schleifer KH (2004) ARB: a software environment for sequence data. Nucleic Acids Res 32:1363–1371. doi:10.1093/nar/gkh293
Malanicheva IA, Kozlov DG, Sumarukova IG, Efremenkova OV, Zenkova VA, Katrukha GS, Reznikova MI, Tarasova OD, Sineokii SP, El'-Registan GI (2012) Antimicrobial activity of Bacillus megaterium strains. Microbiology 81:178–185. doi:10.1134/S0026261712020063
Meerak J, Iida H, Watanabe Y, Miyashita M, Sato H, Nakagawa Y, Tahara Y (2007) Phylogeny of gamma-polyglutamic acid-producing Bacillus strains isolated from fermented soybean foods manufactured in Asian countries. J Gen Appl Microbiol 53:315–323. doi:10.2323/jgam.53.315
Monteiro SM, Clemente JJ, Henriques AO, Gomes RJ, Carrondo MJ, Cunha AE (2005) A procedure for high-yield spore production by Bacillus subtilis. Biotechnol Prog 21:1026–1031. doi:10.1021/bp050062z
Novak KN, Davis E, Wehnes CA, Shields DR, Coalson JA, Smith AH, Rehberger TG (2012) Effect of supplementation with an electrolyte containing a Bacillus-based direct-fed microbial on immune development in dairy calves. Res Vet Sci 92:427–434. doi:10.1016/j.rvsc.2011.04.008
Pattnaik P, Kaushik JK, Grover S, Batish VK (2001) Purification and characterization of a bacteriocin-like compound (Lichenin) produced anaerobically by Bacillus licheniformis isolated from water buffalo. J Appl Microbiol 91:636–645. doi:10.1046/j.1365-2672.2001.01429.x
Pedersen LL, Owusu-Kwarteng J, Thorsen L, Jespersen L (2012) Biodiversity and probiotic potential of yeasts isolated from Fura, a West African spontaneously fermented cereal. Int J Food Microbiol 159:144–151. doi:10.1016/j.ijfoodmicro.2012.08.016
Rooney AP, Price NP, Ehrhardt C, Swezey JL, Bannan JD (2009) Phylogeny and molecular taxonomy of the Bacillus subtilis species complex and description of Bacillus subtilis subsp. inaquosorum subsp. nov. Int J Syst Evol Microbiol 59:2429–2436. doi:10.1099/ijs.0.009126-0
Ruckert C, Blom J, Chen X, Reva O, Borriss R (2011) Genome sequence of B. amyloliquefaciens type strain DSM7(T) reveals differences to plant-associated B. amyloliquefaciens FZB42. J Biotechnol 155:78–85. doi:10.1016/j.jbiotec.2011.01.006
Schallmey M, Singh A, Ward OP (2004) Developments in the use of Bacillus species for industrial production. Can J Microbiol 50:1–17. doi:10.1139/w03-076
Schierack P, Nordhoff M, Pollmann M, Weyrauch KD, Amasheh S, Lodemann U, Jores J, Tachu B, Kleta S, Blikslager A, Tedin K, Wieler LH (2006) Characterization of a porcine intestinal epithelial cell line for in vitro studies of microbial pathogenesis in swine. Histochem. Cell Biol 125:293–305. doi:10.1007/s00418-005-0067-z
Sorokulova IB, Pinchuk IV, Denayrolles M, Osipova IG, Huang JM, Cutting SM, Urdaci MC (2008) The safety of two Bacillus probiotic strains for human use. Dig Dis Sci 53:954–963. doi:10.1007/s10620-007-9959-1
Sun P, Wang JQ, Zhang HT (2010) Effects of Bacillus subtilis natto on performance and immune function of preweaning calves. J Dairy Sci 93:5851–5855. doi:10.3168/jds.2010-3263
Travers RS, Martin PA, Reichelderfer CF (1987) Selective process for efficient isolation of soil Bacillus spp. Appl. Environ. Microbiol 53:1263–1266. PMCID: PMC203852
Wang LT, Lee FL, Tai CJ, Kasai H (2007) Comparison of gyrB gene sequences, 16S rRNA gene sequences and DNA–DNA hybridization in the Bacillus subtilis group. Int J Syst Evol Microbiol 57:1846–1850. doi:10.1099/ijs.0.64685-0
Williams LD, Burdock GA, Jimenez G, Castillo M (2009) Literature review on the safety of Toyocerin, a non-toxigenic and non-pathogenic Bacillus cereus var. toyoi preparation. Regul ToxicolPharmacol 55:236–246. doi:10.1016/j.yrtph.2009.07.009
Xu H, He X, Gou J, Lee HY, Ahn J (2009) Kinetic evaluation of physiological heterogeneity in bacterial spores during thermal inactivation. J Gen Appl Microbiol 55:295–299. doi:10.2323/jgam.55.295
Yu Q, Wang Z, Yang Q (2012) Lactobacillus amylophilus D14 protects tight junction from enteropathogenic bacteria damage in Caco-2 cells. J Dairy Sci 95:5580–5587. doi:10.3168/jds.2012-5540
Acknowledgments
This work was financially supported by Chr. Hansen A/S (Denmark) and the Danish International Development Agency (DANIDA) Seedfood project. The excellent assistance of laboratory technicians Abdallah Albayasli, Nina Milora and Jonna Nielsen (Chr. Hansen A/S) is gratefully acknowledged. Thanks to Animal Health and Veterinary Laboratory Agencies (AHVLA, UK) as well as Royal Holloway University of London for supplying selected Bacillus strains.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 33 kb)
Rights and permissions
About this article
Cite this article
Larsen, N., Thorsen, L., Kpikpi, E.N. et al. Characterization of Bacillus spp. strains for use as probiotic additives in pig feed. Appl Microbiol Biotechnol 98, 1105–1118 (2014). https://doi.org/10.1007/s00253-013-5343-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-5343-6