Abstract
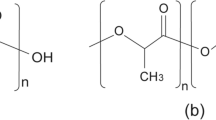
The importance of polylactic acid, a representative bio-based polyester, has been established on a worldwide scale in response to emerging global environmental problems such as green house gas emission and limited petroleum consumption. The current methods for generating this bio-based polymer involve biological synthesis and lactic acid (LA) fermentation, followed by chemical ring-opening polymerization. Among the research community working on polyhydroxyalkanoate polyesters, the prospect of direct biological synthesis of LA into a polymeric form is very attractive from the academic and industrial perspectives. In 2008, this challenge was met for the first time by the discovery of an “LA-polymerizing enzyme”. Using this novel enzyme, the metabolic engineering approach outlined here provided an entirely new, single organism generation of the polymer. This is a major breakthrough in the field. In this review, we provide an overview of the whole-cell synthesis of LA-containing polyesters in comparison with conventional lipase-catalyzed polymer synthesis in terms of both the concepts and strategies of their synthetic processes.







Similar content being viewed by others
References
Abe H, Doi Y, Hori Y, Hagiwara T (1998) Physical properties and enzymatic degradability of copolymers of (R)-3-hydroxybutyric acid and (S,S)-lactide. Polymer 39:59–67
Albertsson AC, Edlund U, Stridsberg K (2000) Controlled ring-opening polymerization of lactones and lactides. Macromol Symp 157:39–46
Aoyagi Y, Doi Y, Iwata T (2003) Mechanical properties and highly ordered structure of ultra-high-molecular-weight poly[(R)-3-hydroxybutyrate] films: effects of annealing and two-step drawing. Polym Degrad Stab 79:209–216
Auras R, Harte B, Selke S (2004) An overview of polylactides as packaging materials. Macromol Biosci 4:835–864
Davis RC, Stahnke G, Wong H, Doolittle MH, Ameis D, Will H, Schotz MC (1990) Hepatic lipase: site-directed mutagenesis of a serine residue important for catalytic activity. J Biol Chem 265:6291–6295
Doi Y (1990) Microbial Polyesters. VHC Publishers, New York
Fijten MWM, Kranenburg JM, Thijs HML, Paulus RM, van Lankvelt BM, de Hullu J, Springintveld M, Thielen DJG, Tweedie CA, Hoogenboom R, Van Vliet KJ, Schubert US (2007) Synthesis and structure-property relationships of random and block copolymers: a direct comparison for copoly(2-oxazoline)s. Macromolecules 40:5879–5886
Haywood GW, Anderson AJ, Dawes EA (1989) The importance of PHB-synthase substrate specificity in polyhydroxyalkanoate synthesis by Alcaligenes eutrophus. FEMS Microbiol Lett 57:1–6
Hein S, Paletta JRJ, Steinbüchel A (2002) Cloning, characterization and comparison of the Pseudomonas mendocina polyhydroxyalkanoate synthases PhaC1 and PhaC2. Appl Microbiol Biotechnol 58:229–236
Huisman GW, Wonink E, Meima R, Kazemier B, Terpstra P, Witholt B (1991) Metabolism of poly(3-hydroxyalkanoates) (PHAs) by Pseudomonas oleovorans. Identification and sequences of genes and function of the encoded proteins in the synthesis and degradation of PHA. J Biol Chem 266:2191–2198
Jo SJ, Maeda M, Ooi T, Taguchi S (2006) Production system for biodegradable polyester polyhydroxybutyrate by Corynebacterium glutamicum. J Biosci Bioeng 102:233–236
Jo SJ, Matsumoto K, Leong CR, Ooi T, Taguchi S (2007) Improvement of poly(3-hydroxybutyrate) [P(3HB)] production in Corynebacterium glutamicum by codon optimization, point mutation and gene dosage of P(3HB) biosynthetic genes. J Biosci Bioeng 104:457–463
Kichise T, Taguchi S, Doi Y (2002) Enhanced accumulation and changed monomer composition in polyhydroxyalkanoate (PHA) copolyester by in vitro evolution of Aeromonas caviae PHA synthase. Appl Environ Microbiol 68:2411–2419
Kikuchi H, Uyama H, Kobayashi S (2000) Lipase-catalyzed enantioselective copolymerization of substituted lactones to optically active polyesters. Macromolecules 33:8971–8975
Kobayashi S (2009) Recent developments in lipase-catalyzed synthesis of polyesters. Macromol Rapid Commun 30:237–266
Kricheldorf HR (2001) Syntheses and application of polylactides. Chemosphere 43:49–54
Kumar A, Kalra B, Dekhterman A, Gross RA (2000) Efficient ring-opening polymerization and copolymerization of epsilon-caprolactone and omega-pentadecalactone catalyzed by Candida antartica lipase B. Macromolecules 33:6303–6309
Liebergesell M, Steinbüchel A (1992) Cloning and nucleotide sequences of genes relevant for biosynthesis of poly(3-hydroxybutyric acid) in Chromatium vinosum strain D. Eur J Biochem 209:135–150
Liebergesell M, Sonomoto K, Madkour M, Mayer F, Steinbüchel A (1994) Purification and characterization of the poly(hydroxyalkanoic acid) synthase from Chromatium vinosum and localization of the enzyme at the surface of poly(hydroxyalkanoic acid) granules. Eur J Biochem 226:71–80
Matsumoto K, Matsusaki H, Taguchi K, Seki M, Doi Y (2002) Isolation and characterization of polyhydroxyalkanoates inclusions and their associated proteins in Pseudomonas sp. 61-3. Biomacromolecules 3:787–792
Matsumoto K, Takase K, Aoki E, Doi Y, Taguchi S (2005) Synergistic effects of Glu130Asp substitution in the Type II Polyhydroxyalkanoate (PHA) synthase: enhancement of PHA production and alteration of polymer molecular weight. Biomacromolecules 6:99–104
Matsumoto K, Aoki E, Takase K, Doi Y, Taguchi S (2006) In vivo and in vitro characterization of Ser477X mutations in polyhydroxyalkanoate (PHA) synthase 1 from Pseudomonas sp. 61-3: effects of beneficial mutations on enzymatic activity, substrate specificity, and molecular weight of PHA. Biomacromolecules 7:2436–2442
Matsumoto K, Takase K, Yamamoto Y, Doi Y, Taguchi S (2009) Chimeric enzyme composed of polyhydroxyalkanoate (PHA) synthases from Ralstonia eutropha and Aeromonas caviae enhances production of PHAs in recombinant Escherichia coli. Biomacromolecules 10:682–685
Matsumura S, Mabuchi K, Toshima K (1997) Lipase-catalyzed ring-opening polymerization of lactide. Macromol Rapid Commun 18:477–482
Matsusaki H, Manji S, Taguchi K, Kato M, Fukui T, Doi Y (1998) Cloning and molecular analysis of the poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyalkanoate) biosynthesis genes in Pseudomonas sp. strain 61-3. J Bacteriol 180:6459–6467
Matsusaki H, Abe H, Doi Y (2000) Biosynthesis and properties of poly(3-hydroxybutyrate-co-3-hydroxyalkanoates) by recombinant strains of Pseudomonas sp. 61-3. Biomacromolecules 1:17–22
McCool GJ, Cannon MC (2001) PhaC and PhaR are required for polyhydroxyalkanoic acid synthase activity in Bacillus megaterium. J Bacteriol 183:4235–4243
Mifune J, Grage K, Rehm BHA (2009) Production of functionalized biopolyester granules by recombinant Lactococcus lactis. Appl Environ Microbiol 75:4668–4675
Müh U, Sinskey AJ, Kirby DP, Lane WS, Stubbe J (1999) PHA synthase from Chromatium vinosum: cysteine 149 is involved in covalent catalysis. Biochemistry 38:826–837
Nomura CT, Taguchi S (2007) PHA synthase engineering toward superbiocatalysts for custom-made biopolymers. Appl Microbiol Biotechnol 73:969–979
Normi YM, Hiraishi T, Taguchi S, Sudesh K, Najimudin N, Doi Y (2005) Site-directed saturation mutagenesis at residue F420 and recombination with another beneficial mutation of Ralstonia eutropha polyhydroxyalkanoate synthase. Biotechnol Lett 27:705–712
Ozkoc G, Kemaloglu S (2009) Morphology, biodegradability, mechanical, and thermal properties of nanocomposite films based on PLA and plasticized PLA. J Appl Polym Sci 114:2481–2487
Pillin I, Montrelay N, Grohens Y (2006) Thermo-mechanical characterization of plasticized PLA: is the miscibility the only significant factor? Polymer 47:4676–4682
Rehm BHA (2003) Polyester synthases: natural catalysts for plastics. Biochem J 376:15–33
Satoh Y, Minamoto N, Tajima K, Munekata M (2002) Polyhydroxyalkanoate synthase from Bacillus sp. INT005 is composed of PhaC and PhaR. J Biosci Bioeng 94:343–350
Schubert P, Steinbüchel A, Schlegel HG (1988) Cloning of the Alcaligenes eutrophus genes for synthesis of poly-b-hydroxybutyric acid (PHB) and synthesis of PHB in Escherichia coli. J Bacteriol 170:5837–5847
Schweiger G, Buckel W (1984) On the dehydration of (R)-lactate in the fermentation of alanine to propionate by Clostridium-Propionicum. FEBS Lett 171:79–84
Selmer T, Willanzheimer A, Hetzel M (2002) Propionate CoA-transferase from Clostridium propionicum—cloning of the gene and identification of glutamate 324 at the active site. Eur J Biochem 269:372–380
Sheu DS, Lee CY (2004) Altering the substrate specificity of polyhydroxyalkanoate synthase 1 derived from Pseudomonas putida GPo1 by localized semirandom mutagenesis. J Bacteriol 186:4177–4184
Shozui F, Matsumoto K, Nakai T, Yamada M, Taguchi S (2009a) Biosynthesis of novel terpolymers poly(lactate-co-3-hydroxybutyrate-co-3-hydroxyvalerate)s in lactate-overproducing mutant Escherichia coli JW0885 by feeding propionate as a precursor of 3-hydroxyvalerate. Appl Microbiol Biotechnol doi:10.1007/s00253-009-2100-y
Shozui F, Matsumoto K, Sasaki T, Taguchi S (2009b) Engineering of polyhydroxyalkanoate synthase by Ser477X/Gln481X saturation mutagenesis for efficient production of 3-hydroxybutyrate-based copolyesters. Appl Microbiol Biotechnol 84:1117–1124
Stubbe J, Tian J, He A, Sinskey AJ, Lawrence AG, Liu P (2005) Nontemplate-dependent polymerization processes: polyhydroxyalkanoate synthases as a paradigm. Annu Rev Biochem 74:433–480
Suzuki Y, Taguchi S, Saito T, Toshima K, Matsumura S, Doi Y (2001) Involvement of catalytic amino acid residues in enzyme-catalyzed polymerization for the synthesis of polyesters. Biomacromolecules 2:541–544
Taguchi S, Doi Y (2004) Evolution of polyhydroxyalkanoate (PHA) production system by “enzyme evolution”: successful case studies of directed evolution. Macromol Biosci 4:145–156
Taguchi S, Tsuge T (2008) Natural polyester-realated proteins: structure, function, evolution and engineering. In: Lutz S, Bornschuer UT (eds) Protein engineering handbook. Wiley, Weinheim, pp 877–914
Taguchi S, Yamada M, Matsumoto K, Tajima K, Satoh Y, Munekata M, Ohno K, Kohda K, Shimamura T, Kambe H, Obata S (2008) A microbial factory for lactate-based polyesters using a lactate-polymerizing enzyme. Proc Natl Acad Sci U S A 105:17323–17327
Tajima K, Satoh Y, Satoh T, Itoh R, Han XR, Taguchi S, Kakuchi T, Munekata M (2009) Chemo-enzymatic synthesis of poly(lactate-co-(3-hydroxybutyrate)) by a lactate-polymerizing enzyme. Macromolecules 42:1985–1989
Takase K, Taguchi S, Doi Y (2003) Enhanced synthesis of poly(3-hydroxybutyrate) in recombinant Escherichia coli by means of error-prone PCR mutagenesis, saturation mutagenesis, and in vitro recombination of the type II polyhydroxyalkanoate synthase gene. J Biochem 133:139–145
Takase K, Matsumoto K, Taguchi S, Doi Y (2004) Alteration of substrate chain-length specificity of type II synthase for polyhydroxyalkanoate biosynthesis by in vitro evolution: in vivo and in vitro enzyme assays. Biomacromolecules 5:480–485
Tokiwa Y, Calabia BP (2006) Biodegradability and biodegradation of poly(lactide). Appl Microbiol Biotechnol 72:244–251
Valentin HE, Steinbüchel A (1994) Application of enzymatically synthesized short-chain-length hydroxy fatty acid coenzyme A thioesters for assay of polyhydroxyalkanoic acid synthases. Appl Microbiol Biotechnol 40:699–709
van Buijtenen J, Van As BAC, Verbruggen M, Roumen L, Vekemans JAJM, Pieterse K, Hilbers PAJ, Hulshof LA, Palmans ARA, Meijer EW (2007) Switching from S- to R-Selectivity in the Candida antarctica lipase B-catalyzed ring-opening of omega-methylated lactones: tuning polymerizations by ring size. J Am Chem Soc 129:7393–7398
Verma ML, Azmi W, Kanwar SS (2008) Microbial lipases: at the interface of aqueous and non-aqueous media. A review. Acta Microbiol Immunol Hung 55:265–294
Yamada M, Matsumoto K, Nakai T, Taguchi S (2009) Microbial production of lactate-enriched poly[(R)-lactate-co-(R)-3-hydroxybutyrate] with novel thermal properties. Biomacromolecules 10:677–681
Younes H, Cohn D (1988) phase-separation in poly(ethylene glycol) poly(lactic acid) blends. Eur Polym J 24:765–773
Yuan W, Jia Y, Tian JM, Snell KD, Muh U, Sinskey AJ, Lambalot RH, Walsh CT, Stubbe J (2001) Class I and III polyhydroxyalkanoate synthases from Ralstonia eutropha and Allochromatium vinosum: characterization and substrate specificity studies. Arch Biochem Biophys 394:87–98
Zhao YM, Wang ZY, Wang J, Mai HZ, Yan B, Yang F (2004) Direct synthesis of poly(D, L-lactic acid) by melt polycondensation and its application in drug delivery. J Appl Polym Sci 91:2143–2150
Acknowledgment
We would like to sincerely thank Prof. A. Steinbüchel for giving us a good opportunity to prepare this Mini-Review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsumoto, K., Taguchi, S. Enzymatic and whole-cell synthesis of lactate-containing polyesters: toward the complete biological production of polylactate. Appl Microbiol Biotechnol 85, 921–932 (2010). https://doi.org/10.1007/s00253-009-2374-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-2374-0