Abstract
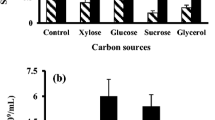
Bacillus atrophaeus’ spores are used in the preparation of bioindicators to monitor the dry heat, ethylene oxide, and plasma sterilization processes and in tests to assess sterilizing products. Earlier production methods involved culture in chemically defined medium to support sporulation with the disadvantage of requiring an extended period of time (14 days) besides high cost of substrates. The effect of cultivation conditions by solid-state fermentation (SSF) was investigated aiming at improving the cost–productivity relation. Initial SSF parameters such as the type of substrate were tested. Process optimization was carried out using factorial experimental designs and response surface methodology in which the influence of different variables—particle size, moisture content, incubation time, pH, inoculum size, calcium sources, and medium composition—was studied. The results have suggested that soybean molasses and sugarcane bagasse are potential substrate and support, respectively, contributing to a 5-day reduction in incubation time. Variables which presented significant effects and optimum values were mean particle size (1.0 mm), moisture content (93%), initial substrate pH (8.0), and water as a solution base. The high-yield spore production was about 3 logs higher than the control and no significant difference in dry heat resistance was observed.




Similar content being viewed by others
References
Association for the Advancement of Medical Instrumentation (AAMI) (2005) ST40:2004. Dry heat (heated air) sterilizers. American National Standard, Arlington
Capalbo DM, Valicente FH, Moraes IO, Pelizer LH (2001) Solid-state fermentation of Bacillus thuringiensis tolworthi to control fall armyworm in maize. Electron J Biotechnol 4:9–10
Cazemier AE, Wagenaars SFM, Steeg PF (2001) Effect of sporulation and recovery medium on the heat resistance and amount of injury of spores from spoilage bacilli. J Appl Microbiol 90:761–777
Chen X, Chen S, Su M, Yu Z (2005) High yield of poly-γ-glutamic acid from Bacillus subtilis by solid-state fermentation using swine manure as the basis of a solid substrate. Bioresour Technol 96:1872–1879
Errington J (2003) Regulation of endospore formation in Bacillus subtilis. Nat Rev Microbiol 1:117–126
Goes AP, Sheppard JD (1999) Effect of surfactants on α-amylase production in a solid substrate fermentation process. J Chem Technol Biotechnol 74:709–712
Gupta S, Kapoor M, Sharma K, Nair L, Kuhad RC (2008) Production and recovery of an alkaline exo-polygalacturonase from Bacillus subtilis RCK under solid-state fermentation using statistical approach. Bioresour Technol 99:937–945
Kashyap DR, Soni SK, Tewari R (2003) Enhanced production of pectinase by Bacillus sp. DT7 using solid state fermentation. Bioresour Technol 88:251–254
Kunamneni A, Singh S (2005) Response surface optimization of enzymatic hydrolysis of maize starch for higher glucose production. Biochem Engin J 27:179–190
Montgomery DC (1977) Response surface methods and other approaches to process optimization. In: Montgomery DC (ed) Design and analysis of experiments. Wiley, New York, pp 427–510
Pandey A, Selvakumar P, Soccol CR, Nigam P (1999) Solid state fermentation for the production of industrial enzymes. Curr Sci 77:149–162
Piggot PJ, Losick R (2002) Sporulation genes and intercompartmental regulation. In: Sonenshein AL, Losick R, Hoch JA (eds) Bacillus subtilis and its closest relatives: from genes to cells. American Society for Microbiology, Washington, DC, pp 483–517
Pinto TJ, Saito T (1992) Esterilização por óxido de etileno. II—Influência de corpos de prova no desempenho de monitores biológicos e sua avaliação. Rev Saúde Pública 26:379–383
Pryor SW, Gibson DM, Hay AG, Gossett JM, Walker LP (2007) Optimization of spore and antifungal lipopeptide production during the solid state fermentation of Bacillus subtilis. Appl Biochem Biotechnol 143:63–79
Redmond C, Baillie LW, Hibbs S, Moir AJ (2004) Identification of proteins in the exosporium of Bacillus anthracis. Microbiology 150:355–363
Schaeffer PJ, Millet J, Aubert JP (1965) Catabolic repression of bacterial sporulation. Proc Natl Acad Sci USA 54:704–711
Sella SRBR, Vandenberghe LPS, Medeiros ABP, Soccol CR (2007) Evaluation of different techniques and culture medium for the Bacillus atrophaeus spores production. Proceedings of the Anais XVI Simpósio Nacional de Bioprocessos, Curitiba, Brazil, CD ISSN/ISBN:9788560328079
Soares VF, Castilho LR, Bon EPS, Freire DMG (2005) High-yield Bacillus subtilis protease production by solid-state fermentation. Appl Biochem Biotechnol 121:311–320
Soccol CR, Vandenberghe LPS (2003) Overview of applied solid-state fermentation in Brazil. Biochem Eng J 13:205–218
Soccol CR, Radjiskumar M, Quorim M (2001) Uso do bagaço de cana de açúcar como material de suporte na fase de enraizamento em processo de micropropagação vegetal. Brazilian patent, NPI No. 931
Sonenshein AL (2000) Control of sporulation initiation in Bacillus subtilis. Curr Opin Microbiol 3:561–566
United States Pharmacopeia XXIX (2005) Biological indicators resistance and performance tests. In: The United States Pharmacopeia, 29th rev. United States Pharmacopoeia Convection Rockville, MD, pp 2501–2503
Veening J (2007) Phenotypic variation in Bacillus subtilis: bistability in the sporulation pathway. Proefschrift Rijksuniversiteit Groningen, Nederland, Print Partners Ipskamp, Enschede
Vries YP, Atmadja RD, Hornstra LM, deVos WM, Abee T (2005) Influence of glutamate on growth, sporulation, and spore properties of Bacillus cereus ATCC 14579 in a defined medium. Appl Environ Microbiol 71:3248–3254
Acknowledgements
This research was financially supported by the Secretaria de Estado da Ciência, Tecnologia e Ensino Superior—Fundo Paraná.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sella, S.R.B.R., Guizelini, B.P., Vandenberghe, L.P.S. et al. Bioindicator production with Bacillus atrophaeus’ thermal-resistant spores cultivated by solid-state fermentation. Appl Microbiol Biotechnol 82, 1019–1026 (2009). https://doi.org/10.1007/s00253-008-1768-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1768-8