Abstract
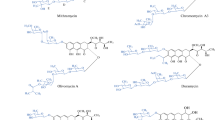
Expression of the aranciamycin biosynthetic gene cluster in Streptomyces diastatochromogenes Tü6028 resulted in production of four novel compounds, aranciamycins E, F, G, and H with different decorations in the tetracyclic backbone. Two derivatives contain a d-amicetose moiety at C7 (aranciamycins F and G), two are hydroxylated at position C1 (aranciamycins E and G), and one is hydroxylated at C13 (aranciamycin F). Analysis of the biological activities of the aranciamycins against two human tumor cell lines—MCF-7 and MATU—shows surprising impact of the hydroxyl group at position C1 on activity. As aranciamycins E and G were the most active derivatives, hydroxylation of the C1 appears to coincide with increased antitumor activity of aranciamycins.



Similar content being viewed by others
References
Arcamone F, Cassinelli G (1998) Biosynthetic anthracyclines. Curr med Chem 5:391–419
Arcamone F, Animati F, Capranico G, Lombardi P, Pratesi G, Manzini S, Supino R, Zunino F (1997) New developments in antitumor anthracyclines. Pharmacol Ther 76:117–24
Beinker P, Lohkamp B, Peltonen T, Niemi J, Mäntsälä P, Schneider G (2006) Crystal structures of SnoaL2 and AclR: two putative hydroxylases in the biosynthesis of aromatic polyketide antibiotics. J Mol Biol 359:728–740
Brodasky TF, Reusser F (1974) Steffimycin B, a new member of the steffimycin family: isolation and characterization. J Antibiot 27:809–813
Bols M, Binderup L, Hansen J, Rasmussen P (1992a) Synthesis and collagenase inhibition of new glycosides of aranciamycinone: the aglycon of the naturally occurring antibiotic aranciamycin. Carbohydr Res 235:141–149
Bols M, Binderup L, Hansen J, Rasmussen P (1992b) Inhibition of collagenase by aranciamycin and aranciamycin derivatives. J Med Chem 35:2768–2771
David L, Duteurtre M, Kergomard A, Kergomard G, Scanzi E, Staron T (1980) Production of cinerubins by a Streptomyces griseorubiginosus strain. J Antibiot 33:49–53
Floss H (2006) Combinatorial biosynthesis–potential and problems. J. Biotechnol 124:242–257
Gianni L, Grasselli G, Cresta S, Locatelli A, Viganò L, Minotti G (2003) Anthracyclines. Cancer Chemother Biol Response Modif 21:29–40
Gullón S, Olano C, Abdelfattah MS, Braña AF, Rohr J, Méndez C, Salas JA (2007) Isolation, characterization, and heterologous expression of the biosynthesis gene cluster for the antitumor anthracycline steffimycin. Appl Environ Microbiol 72:4172–4183
Keller-Schierlein W, Müller A (1970) The sugar component of aranciamycin: 2–0-methyl-L-rhamnose. Experentia 26:929–930
Kratz F, Warnecke A, Schmid B, Chung DE, Gitzel M (2006) Prodrugs of anthracyclines in cancer chemotherapy. Curr Med Chem 5:477–523
Luzhetskyy A, Bechthold A (2005) It works: combinatorial biosynthesis for generating novel glycosylated compounds. Mol Microbiol 58:3–5
Luzhetskyy A, Fedoryshyn M, Dürr C, Taguchi T, Novikov V, Bechthold A (2005) Iteratively acting glycosyltransferases involved in the hexasaccharide biosynthesis of landomycin A. Chem Biol 12:725–729
Luzhetskyy A, Mayer A, Hoffmann J, Pelzer S, Holzenkämper M, Schmitt B, Wohlert SE, Vente A, Bechthold A (2007) Cloning and heterologous expression of the aranciamycin biosynthetic gene cluster revealed a new flexible glycosyltransferase. Chembiochem 6:599–602
Luzhetskyy A, Méndez C, Salas J, Bechthold A (2008) Glycosyltransferases, important tools for drug design. Curr Top Med Chem 8:680–709
Madduri K, Kennedy J, Rivola G, Inventi-Solari A, Filippini S, Zanuso G, Colombo AL, Gewain KM, Occi JL, MacNeil DJ, Hutchinson CR (1998) Production of the antitumor drug epirubicin (4’¢-epidoxorubicin) and its precursor by a genetically engineered strain of Streptomyces peucetius. Nat Biotechnol 16:69–74
Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L (2004) Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev 56:185–229
Olano C, Abdelfattah MS, Gullón S, Braña AF, Rohr J, Méndez C, Salas JA (2008) Glycosylated Derivatives of Steffimycin: Insights into the Role of the Sugar Moieties for the Biological Activity. Chembiochem 3:624–633
Ostash B, Rix U, Rix LL, Liu T, Lombo F, Luzhetskyy A, Gromyko O, Wang C, Braña AF, Méndez C, Salas JA, Fedorenko V, Rohr J (2004) Generation of new landomycins by combinatorial biosynthetic1 manipulation of the LndGT4 gene of the landomycin E cluster in S. globisporus. Chem Biol 11:547–555
Aknowledgment
This work was supported in part by the BMBF (GenoMic-Plus) and by the DFG (Synthese von Dekaketid-Zwischenstufen der Polyketidsynthasen vom TypII), both grants to AB.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
NMR spectra of the aranciamycin H molecule (DOC 65.5 kb)
Figure S2
NMR spectra of the aranciamycin G molecule. (DOC 114 kb)
Figure S3
NMR spectra of the aranciamycin F molecule. (DOC 89.0 kb)
Figure S4
NMR spectra of the aranciamycin E molecule. (DOC 82.0 kb)
Rights and permissions
About this article
Cite this article
Luzhetskyy, A., Hoffmann, J., Pelzer, S. et al. Aranciamycin analogs generated by combinatorial biosynthesis show improved antitumor activity. Appl Microbiol Biotechnol 80, 15–19 (2008). https://doi.org/10.1007/s00253-008-1515-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1515-1