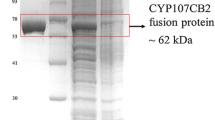
Abstract
Cytochrome P450 monooxygenases of the CYP102A subfamily are single-component natural fusion proteins consisting of a heme domain and a diflavin reductase. The characterised CYP102A enzymes are fatty acid hydroxylases with turnover rates of several thousands per minute. In search of new P450s with similar activities, but with a broader substrate spectrum, we cloned, expressed and characterised CYP102A7 from Bacillus licheniformis. As expected, CYP102A7 was active towards medium-chain fatty acids but showed a strong preference for saturated over unsaturated fatty acids, which could not be observed for either of the CYP102A members so far. Besides fatty acids, CYP102A7 was able to catalyse the oxidation of cyclic and acyclic terpenes with high activity and coupling efficiency. For example, (R)-(+)-limonene was converted with activity of 220 nmol nmol P450−1 min−1 and 80% coupling. Unusual for enzymes of the CYP102A subfamily was the deethylation activity of CYP102A7 towards 7-ethoxycoumarin. Furthermore, this monooxygenase, though having a moderate thermal stability, exhibited 50% of its initial activity in the presence of 26% DMSO. Comparison of the homology models of CYP102A7 and other members of the CYP102A subfamily revealed distinct differences in the shape of the substrate access channel and the active site, which might explain differences in catalytic properties of these homologous enzymes.





Similar content being viewed by others
References
Appel D, Lutz-Wahl S, Fischer P, Schwaneberg U, Schmid RD (2001) A P450BM-3 mutant hydroxylates alkanes, cycloalkanes, arenes and heteroarenes. J Biotechnol 88:167–171
Boddupalli SS, Oster T, Estabrook RW, Peterson JA (1992) Reconstitution of the fatty acid hydroxylation function of cytochrome P450 BM-3 utilizing its individual recombinant hemo- and flavoprotein domains. J Biol Chem 267:10375–10380
Brady GP Jr, Stouten PF (2000) Fast prediction and visualization of protein binding pockets with PASS. J Comput Aided Mol Des 14:383–401
Budde M, Maurer SC, Schmid RD, Urlacher VB (2004) Cloning, expression and characterisation of CYP102A2, a self-sufficient P450 monooxygenase from Bacillus subtilis. Appl Microbiol Biotechnol 66:180–186
Budde M, Morr M, Schmid RD, Urlacher VB (2006) Selective hydroxylation of highly branched fatty acids and their derivatives by CYP102A1 from Bacillus megaterium. Chembiochem 7:789–794
Capdevila JH, Wei S, Helvig C, Falck JR, Belosludtsev Y, Truan G, Graham-Lorence SE, Peterson JA (1996) The highly stereoselective oxidation of polyunsaturated fatty acids by cytochrome P450 BM-3. J Biol Chem 271:22663–22671
Chowdhary PK, Alemseghed M, Haines DC (2007a) Cloning, expression and characterization of a fast self-sufficient P450: CYP102A5 from Bacillus cereus. Arch Biochem Biophys 468:32–43
Chowdhary PK, Keshavan N, Nguyen HQ, Peterson JA, Gonzalez JE, Haines DC (2007b) Bacillus megaterium CYP102A1 oxidation of acyl homoserine lactones and acyl homoserines. Biochemistry 46:14429–14437
DeLano WL (2005) The case for open-source software in drug discovery. Drug Discov Today 10:213–217
Eiben S, Bartelmas H, Urlacher VB (2007) Construction of a thermostable cytochrome P450 chimera derived from self-sufficient mesophilic parents. Appl Microbiol Biotechnol 75:1055–1061
Guex N, Peitsch MC (1997) SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18:2714–2723
Gustafsson MC, Roitel O, Marshall KR, Noble MA, Chapman SK, Pessegueiro A, Fulco AJ, Cheesman MR, von Wachenfeldt C, Munro AW (2004) Expression, purification, and characterization of Bacillus subtilis cytochromes P450 CYP102A2 and CYP102A3: flavocytochrome homologues of P450 BM3 from Bacillus megaterium. Biochemistry 43:5474–5487
Haines DC, Tomchick DR, Machius M, Peterson JA (2001) Pivotal role of water in the mechanism of P450BM-3. Biochemistry 40:13456–13465
Hannemann F, Bichet A, Ewen KM, Bernhardt R (2007) Cytochrome P450 systems–biological variations of electron transport chains. Biochim Biophys Acta 1770:330–344
Isin EM, Guengerich FP (2007) Complex reactions catalyzed by cytochrome P450 enzymes. Biochim Biophys Acta 1770:314–329
Joyce MG, Girvan HM, Munro AW, Leys D (2004) A single mutation in cytochrome P450 BM3 induces the conformational rearrangement seen upon substrate binding in the wild-type enzyme. J Biol Chem 279:23287–23293
Kim BS, Kim SY, Park J, Park W, Hwang KY, Yoon YJ, Oh WK, Kim BY, Ahn JS (2007) Sequence-based screening for self-sufficient P450 monooxygenase from a metagenome library. J Appl Microbiol 102:1392–1400
Kuehnel K, Maurer SC, Galeyeva Y, Frey W, Laschat S, Urlacher VB (2007) Hydroxylation of dodecanoic acid and (2R,4R,6R,8R)-tetramethyldecanol on a preparative scale using an NADH-dependent CYP102A1 mutant. Adv Synth Catal 349:1451–1461
Lentz O, Urlacher V, Schmid RD (2004) Substrate specificity of native and mutated cytochrome P450 (CYP102A3) from Bacillus subtilis. J Biotechnol 108:41–49
Li H, Poulos TL (1997) The structure of the cytochrome P450BM-3 haem domain complexed with the fatty acid substrate, palmitoleic acid. Nat Struct Biol 4:140–146
Liu L, Schmid RD, Urlacher VB (2006) Cloning, expression, and characterization of a self-sufficient cytochrome P450 monooxygenase from Rhodococcus ruber DSM 44319. Appl Microbiol Biotechnol 72:876–882
Maurer S, Urlacher V, Schulze H, Schmid RD (2003) Immobilisation of P450 BM-3 and an NADP+ cofactor recycling system: towards a technical application of heme-containing monooxygenases in fine chemical synthesis. Adv Synth Catal 345:802–810
Narhi LO, Fulco AJ (1987) Identification and characterization of 2 functional domains in cytochrome P-450BM-3, a catalytically self-sufficient monooxygenase induced by barbiturates in Bacillus megaterium. J Biol Chem 262:6683–6690
Narhi LO, Wen LP, Fulco AJ (1988) Characterization of the protein expressed in Escherichia coli by a recombinant plasmid containing the Bacillus megaterium cytochrome P-450BM-3 gene. Mol Cell Biochem 79:63–71
Noble MA, Miles CS, Chapman SK, Lysek DA, MacKay AC, Reid GA, Hanzlik RP, Munro AW (1999) Roles of key active-site residues in flavocytochrome P450 BM3. Biochem J 339(Pt 2):371–379
Omura T, Sato R (1964) The carbon monoxide-binding pigment of liver microsomes. II. Solubilization, purification, and properties. J Biol Chem 239:2379–2385
Ortiz de Montellano P (2005) Cytochrome P450: structure, mechanism and biochemistry, 3rd edn. Springer, The Netherlands
Ost TW, Munro AW, Mowat CG, Taylor PR, Pesseguiero A, Fulco AJ, Cho AK, Cheesman MA, Walkinshaw MD, Chapman SK (2001) Structural and spectroscopic analysis of the F393H mutant of flavocytochrome P450 BM3. Biochemistry 40:13430–13438
Ravichandran KG, Boddupalli SS, Hasemann CA, Peterson JA, Deisenhofer J (1993) Crystal structure of hemoprotein domain of P450BM-3, a prototype for microsomal P450s. Science 261:731–736
Roberts GA, Celik A, Hunter DJ, Ost TW, White JH, Chapman SK, Turner NJ, Flitsch SL (2003) A self-sufficient cytochrome P450 with a primary structural organization that includes a flavin domain and a [2Fe-2S] redox center. J Biol Chem 278:48914–48920
Schwaneberg U, Schmidt-Dannert C, Schmitt J, Schmid RD (1999) A continuous spectrophotometric assay for P450 BM-3, a fatty acid hydroxylating enzyme, and its mutant F87A. Anal Biochem 269:359–366
Schwede T, Kopp J, Guex N, Peitsch MC (2003) SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res 31:3381–3385
Sowden RJ, Yasmin S, Rees NH, Bell SG, Wong LL (2005) Biotransformation of the sesquiterpene (+)-valencene by cytochrome P450cam and P450BM-3. Org Biomol Chem 3:57–64
Urlacher VB, Makhsumkhanov A, Schmid RD (2006) Biotransformation of beta-ionone by engineered cytochrome P450 BM-3. Appl Microbiol Biotechnol 70:53–59
Watanabe Y, Laschat S, Budde M, Affolter O, Shimada Y, Urlacher VB (2007) Oxidation of acyclic monoterpenes by P450 BM-3 monooxygenase: influence of the substrate E/Z-isomerism on enzyme chemo- and regioselectivity. Tetrahedron 63:9413–9422
Wen LP, Fulco AJ (1987) Cloning of the gene encoding a catalytically self-sufficient cytochrome P-450 fatty acid monooxygenase induced by barbiturates in Bacillus megaterium and its functional expression and regulation in heterologous (Escherichia coli) and homologous (Bacillus megaterium) hosts. J Biol Chem 262:6676–6682
Acknowledgements
We thank Peter Staffeld for technical assistance. T.A.D. acknowledges financial support from German Academic Exchange Service (DAAD, Bonn, Germany). The authors acknowledge a grant of the Deutsche Forschungsgemeinschaft (SFB 706) and the Ministerium für Wissenschaft, Forschung und Kunst des Landes Baden-Württemberg and the Fonds der Chemie.
Author information
Authors and Affiliations
Corresponding author
Additional information
M. Dietrich and S. Eiben contributed equally to this work.
Rights and permissions
About this article
Cite this article
Dietrich, M., Eiben, S., Asta, C. et al. Cloning, expression and characterisation of CYP102A7, a self-sufficient P450 monooxygenase from Bacillus licheniformis . Appl Microbiol Biotechnol 79, 931–940 (2008). https://doi.org/10.1007/s00253-008-1500-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1500-8