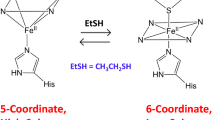
Abstract
NADH peroxidase (Npx) and mercuric ion reductase (MerA) are flavoproteins belonging to the pyridine nucleotide:disulfide oxidoreductases (PNDO) and catalyzing the reduction of toxic substrates, i.e., hydrogen peroxide and mercuric ion, respectively. To determine the role of the flavin adenine dinucleotide (FAD) in the detoxification mechanism, the resonance Raman (RR) spectra of these enzymes under various redox and ligation states have been investigated using blue and/or near-UV excitation(s). These data were compared to those previously obtained for glutathione reductase (GR), another enzyme of the PNDO family, but catalyzing the reduction of oxidized glutathione. Spectral differences have been detected for the marker bands of the isoalloxazine ring of Npx, MerA, and GR. They provide evidence for different catalytic mechanisms in these flavoproteins. The RR modes of the oxidized and two-electron reduced (EH2) forms of Npx are related to very tight flavin–protein interactions maintaining a nearly planar conformation of the isoalloxazine tricycle, a low level of H-bonding at the N1/N5 and O2/O4 sites, and a strong H-bond at N3H. They also indicate minimal changes in FAD structure and environment upon either NAD(H) binding or reduction of the sulfinic redox center. All these spectroscopic data support an enzyme functioning centered on the Cys-SO−/Cys-S− redox moiety and a neighbouring His residue. On the contrary, the RR data on various functional forms of MerA are indicative of a modulation of both ring II distortion and H-bonding states of the N5 site and ring III. The Cd(II) binding to the EH2–NADP(H) complexes, biomimetic intermediates in the reaction of Hg(II) reduction, provokes important spectral changes. They are interpreted in terms of flattening of the isoalloxazine ring and large decreases in H-bonding at the N5 site and ring III. The large flexibility of the FAD structure and environment in MerA is in agreement with proposed mechanisms involving C4a(flavin) adducts.





The vibrational data are from Dutta et al. (1978), Nishina et al. (1978, 1980, 1981, 1992, 2001), Kitagawa et al. (1979, 1982), Benecky et al. (1979), Irwin et al. (1980), Bowman and Spiro (1981), Williamson et al. (1982), Schmidt et al. (1981, 1983), Visser et al. (1983), Sugiyama et al. (1985), Copeland and Spiro (1986), Abe and Kyogoku (1987), Desbois et al. (1989), Kim and Carey (1993), Hazekawa el al. (1997), Tegoni et al. (1997), Macdonald et al. (1999), Zheng et al. (1999), Altose et al. (2001), Picaud and Desbois (2002, 2006), Zheng (2002), Ataka et al. (2003), Unno et al. (2005, 2012), Kottke et al. (2006), Li et al. (2006a, b), Schelvis et al. (2006), Yang and Swenson (2007), Eisenberg and Schelvis (2008), Alexandre et al. (2008, 2010), Kikuchi et al. (2009), Røhr et al. (2010), Weigel et al. (2011), Hikita et al. (2013)



Similar content being viewed by others
Abbreviations
- PNDO:
-
Pyridine nucleotide:disulfide oxidoreductase
- Npx:
-
NADH peroxidase
- MerA:
-
Mercuric ion reductase
- GR:
-
Glutathione reductase
- BLUF:
-
Blue-light-using FAD
- pHBH:
-
p-Hydroxybenzoate hydroxylase
- ETF:
-
Electron-transfer flavoprotein
- Eox :
-
Oxidized form of Npx, MerA, or GR
- EH2 :
-
Two-electron reduced form of Npx, MerA or GR
- Ef :
-
Enterococcus faecalis
- Rm :
-
Ralstonia metallidurans
- Ec :
-
Escherichia coli
- Pa :
-
Pseudomonas aeruginosa
- Lf:
-
Lumiflavin
- Rf:
-
Riboflavin
- FMN:
-
Flavin mononucleotide
- FAD:
-
Flavin adenine dinucleotide
- Me:
-
Methyl
- Rib:
-
Ribityl
- Cys-SH:
-
Cysteine-thiol
- Cys-S− :
-
Cysteine-thiolate
- Cys-SOH:
-
Cysteine-sulfenic acid
- Cys-SO− :
-
Cysteine-sulfenate
- Cys-SO2H:
-
Cysteine-sulfinic acid
- Cys-SO3H:
-
Cysteine-sulfonic acid
- DTT:
-
1,4-Dithiothreitol
- GSH:
-
Reduced glutathione
- eq:
-
Equivalent
- RR:
-
Resonance Raman
- CT:
-
Charge transfer
References
Abe M, Kyogoku Y (1987) Vibrational analysis of flavin derivatives: normal coordinate treatments of lumiflavin. Spectrochim Acta 43A:1027–1037
Abe M, Kyogoku Y, Kitagawa T, Kawano N, Ohishi A, Takai-Suzuki A, Yagi K (1986) Infrared spectra and molecular association of lumiflavin and riboflavin derivatives. Spectrochim Acta 42A:1059–1068
Alexandre MTA, van Grondelle R, Hellingwerf KJ, Robert B, Kennis JTM (2008) Perturbation of the ground-state electronic structure of FMN by the conserved cysteine in phototropin LOV2 domains. Phys Chem Chem Phys 10:6693–6702
Alexandre MTA, Purcell EB, van Grondelle R, Robert B, Kennis JTM, Crosson S (2010) Electronic and protein structural dynamics of a photosensory histidine kinase. Biochemistry 49:4752–4759
Altose MD, Zheng Y, Dong J, Palfey BA, Carey PR (2001) Comparing protein-ligand interactions in solution and single crystals by Raman spectroscopy. Proc Natl Acad Sci USA 28:3006–3011
Anderson S, Dragnea V, Masuda S, Ybe J, Moffat K, Bauer C (2005) Structure of a novel photoreceptor, the BLUF domain of AppA from Rhodobacter sphaeroides. Biochemistry 44:7998–8005
Argyrou A, Blanchard JS (2004) Flavoprotein disulfide reductases: advances in chemistry and function. Prog Nucleic Acid Res Mol Biol 78:89–142
Ataka K, Hegeman P, Heberle J (2003) Vibrational spectroscopy of an algal Phot-LOV1 domain probes the molecular changes associated with blue-light reception. Biophys J 84:466–474
Benecky M, Li TY, Schmidt J, Frerman F, Watters KL, Mc Farland J (1979) Resonance Raman study of flavins and the flavoprotein fatty acyl coenzyme A dehydrogenase. Biochemistry 18:3471–3476
Berkholz DS, Faber HR, Savvides SN, Karplus PA (2008) Catalytic cycle of human glutathione reductase near 1 Å resolution. J Mol Biol 382:371–384
Blaghen M, Vidon DJM, El Kebbaj MS (1993) Purification and properties of mercuric reductase from Yersinia enterocolitica. Can J Microbiol 39:193–200
Bowman WD, Spiro TG (1981) Normal mode analysis of lumiflavin and interpretation of resonance Raman spectra of flavoproteins. Biochemistry 20:3313–3318
Brown NL, Ford SJ, Fridmore RD, Fritzinger DC (1983) Deoxyribonucleic acid sequence of a gene from the Pseudomonas transposon TN501 encoding mercuric reductase. Biochemistry 22:4089–4095
Champier L (2003) Caractérisation des protéines de régulation impliquées dans la résistance au mercure inorganique chez Ralstonia metallidurans CH34. Thesis, University of Grenoble 1, France
Copeland RA, Spiro TG (1986) Ultraviolet resonance Raman spectroscopy of flavin mononucleotide and flavin adenine dinucleotide. J Phys Chem 90:6648–6654
Crane EJ, Parsonage D, Poole LB, Claiborne A (1995) Analysis of the kinetic mechanism of enterococcal NADH peroxidase reveals catalytic roles for NADH complexes with both oxidized and two-electron-reduced enzyme forms. Biochemistry 34:14114–14124
Desbois A, Tegoni M, Gervais M, Lutz M (1989) Flavin and heme structure in lactate–cytochrome c oxidoreductase. A resonance Raman study. Biochemistry 28:8011–8022
Djordjevic S, Pace CP, Stankovich MT, Kim JJP (1995) Three-dimensional structure of butyryl-CoA dehydrogenase from Megasphaera elsdenii. Biochemistry 34:2163–2171
Dutta PK, Nestor JR, Spiro TG (1978) Resonance coherent anti-Stokes Raman scattering (CARS) spectra of flavin adenine dinucleotide riboflabin binding protein and glucose oxidase. Biochem Biophys Res Commun 83:209–216
Eisenberg AS, Schelvis JPM (2008) Contributions of the 8-methyl group in the vibrational normal modes of flavin mononucleotide and its 5-methyl semiquinone radical. J Phys Chem A 112:6179–6189
Engst S, Miller SM (1998) Rapid reduction of Hg(II) by mercuric ion reductase does not require the conserved C-terminal cysteine pair using HgBr 2 as the substrate. Biochemistry 37:11496–11507
Engst S, Miller SM (1999) Alternative routes for entry of HgX2 into the active site of mercuric ion reductase depend on the nature of the X ligands. Biochemistry 38:3519–3529
Enroth C, Neujahr H, Schneider G, Lindqvist Y (1998) The crystal structure of phenyl hydroxylase in complex with FAD and phenol provides evidence for a concerted conformational change in the enzyme and its cofactor during catalysis. Structure 6:605–717
Fox KM, Karplus PA (1999) The flavin environment in old yellow enzyme. An evaluation of insights from spectroscopic and artificial flavin studies. J Biol Chem 274:9357–9362
Fox BS, Walsh CT (1982) Mercuric reductase: purification and characterization of a transposon-encoded flavoprotein containing an oxidation–reduction-active disulfide. J Biol Chem 257:2498–2503
Fox BS, Walsh CT (1983) Mercuric reductase: homology to glutathione reductase and lipoamide dehydrogenase. Iodoacetamide alkylation and sequence of the active site peptide. Biochemistry 22:4082–4088
Gatti DL, Palfey BA, Lah MS, Entsch B, Massey V, Ballou DP, Ludwig ML (1994) The mobile flavin of 4-OH benzoate hydroxylase. Science 266:110–114
Hazekawa I, Nishina Y, Sato K, Shichiri M, Miura R, Shiga KA (1997) Raman study on the C(4)=O stretching mode of flavins in flavoenzymes: hydrogen bonding at the C(4)=O moiety. J Biochem 121:1147–1154
Hecht HJ, Kalisz HM, Hendle J, Schmid RD, Schomburg D (1993) Crystal structure of glucose oxidase from Aspergillus niger refined at 2.3 Å resolution. J Mol Biol 229:153–172
Hikita M, Shinzawa-Itoh K, Moriyama M, Ogura T, Kihira K, Yoshikawa S (2013) Resonance Raman spectral properties of FMN of bovine heart NADH:ubiquinone oxidoreductase suggesting a mechanism for the prevention of spontaneous production of reactive oxygen species. Biochemistry 52:98–104
Hubbard PA, Shen AL, Paschke R, Kasper CB, Kim JJP (2001) NADPH-cytochrome P450 oxidoreductase: structural basis for hydride and electron transfer. J Biol Chem 276:29163–29170
Irwin RM, Visser AJWG, Lee J, Carreira LA (1980) Protein-ligand interactions in lumazine protein and in Desulfovibrio flavodoxins from resonance coherent anti-Stokes Raman spectra. Biochemistry 19:4639–4646
Johansson R, Torrenta E, Lundin D, Sprenger J, Sahlin M, Sjöberg BM, Logan DT (2010) High-resolution crystal structures of the flavoprotein NrdI in oxidized and reduced states. An unusual flavodoxin. FEBS J 277:4265–4277
Johs A, Harwood IM, Parks JM, Nauss RE, Smith JC, Liang L, Miller S (2011) Structural Characterization of intramolecular Hg2+ transfer between flexibly linked domains of mercuric ion reductase. J Mol Biol 413:639–656
Karplus PA, Schulz GE (1987) Refined structure of glutathione reductase at 1.54 Å resolution. J Mol Biol 195:701–729
Kikuchi S, Unno M, Zikihara K, Tokutomi S, Yamauchi S (2009) Vibrational assignment of the flavin–cysteinyl adduct in a signaling state of the LOV domain in FKF1. J Phys Chem B 113:2913–2921
Kim M, Carey PR (1993) Observation of a carbonyl feature for riboflavin bound to riboflavin-binding protein in the red-excited Raman spectrum. J Am Chem Soc 115:7015–7016
Kim JJP, Wang M, Paschke R (1993) Crystal structure of medium-chain acyl-CoA dehydrogenase from pig liver mitochondria with and without substrate. Proc Nat Acad Sci USA 90:7523–7527
Kitagawa T, Nishina Y, Kyokoku Y, Yamano T, Ohishi N, Takai-Suzuki A, Yagi K (1979) Resonance Raman spectra of carbon-13 and nitrogen-15-labeled riboflavin bound to egg-white flavoprotein. Biochemistry 18:1804–1808
Kitagawa T, Sakamoto H, Sugiyama T, Yamano T (1982) Formation of the semiquinone form in the anaerobic reduction of adrenodoxin reductase by NADPH. Resonance Raman, EPR, and optical spectroscopic evidence. J Biol Chem 257:12075–12080
Klaumünzer B, Kröner D, Saalfrank P (2010) (TD-)DFT calculation of vibrational and vibronic spectra of riboflavin in solution. J Phys Chem B 114:10826–10834
Kottke T, Batschauer A, Ahmad M, Heberle J (2006) Blue-light-induced changes in Arabidopsis cryptochrome 1 probed by FTIR difference spectroscopy. Biochemistry 45:2472–2479
Ledwidge R, Patel B, Dong A, Fiedler D, Falkowski M, Zelikova J, Summers AO, Pai EF, Miller SM (2005) NmerA, the metal binding domain of mercuric ion reductase, remove Hg2+ from proteins, delivers it to the catalytic core, and protects cells under glutathione-depleted conditions. Biochemistry 44:11402–11416
Leys D, Basran J, Talfournier F, Sutcliffe MJ, Scrutton NS (2003) Extensive conformational sampling in a ternary electron transfer complex. Nat Struct Biol 10:219–225
Li J, Uchida T, Ohta T, Todo T, Kitagawa T (2006a) Characteristic structure and environment in FAD cofactor of (6-4) photolyase along function revealed by resonance Raman spectroscopy. J Phys Chem B 110:16724–16732
Li J, Uchida T, Todo T, Kitagawa T (2006b) Similarities and differences between cyclobutane pyrimidine dimer photolyase and (6-4) photolyase as revealed by resonance Raman spectroscopy. Electron transfer from the FAD cofactor to ultraviolet-damaged DNA. J Biol Chem 281:25551–25559
Lian P, Guo HB, Riccardi D, Dong A, Parks JM, Xu Q, Pai EF, Miller SM, Wei DQ, Smith JC, Guo H (2014) X-Ray structure of a Hg2+ complex of mercuric reductase (MerA) and quantum mechanical/molecular mechanical study of Hg2+ transfer between the C-terminal and buried catalytic site cysteine pairs. Biochemistry 53:7211–7222
Lin LYC, Sulea T, Szittner R, Vassilyev V, Purisima EO, Meighen EA (2001) Modeling of the bacterial luciferase–flavin mononucleotide complex combining flexible docking with structure–activity data. Protein Sci 10:1563–1571
Lively CR, McFarland JT (1990) Assignment and the effect of hydrogen bonding on the vibrational normal modes of flavins and flavoproteins. J Phys Chem 94:3980–3994
Macdonald IDG, Smith WE, Munro AW (1999) Analysis of the structure of the flavin-binding sites of flavocytochrome P450 BM3 using surface enhanced resonance Raman scattering. Eur Biophys J 28:437–445
Marshall JL, Booth JE, Williams JW (1984) Characterization of the covalent mercury(II)–NADPH complexes. J Biol Chem 259:3033–3036
Mattevi A, Vanoni MA, Todone F, Rizzi M, Teplyakov A, Coda A, Bolognesi M, Curti B (1996) Crystal structure of d-amino acid oxidase: a case of active site mirror-image convergent evolution with flavocytochrome b2. Proc Natl Acad Sci USA 93:7496–7501
Miller S, Moore MJ, Massey V, Williams CH Jr, Distefano MD, Ballou DP, Walsh CT (1989) Evidence for the participation of Cys558 and Cys559 at the active site of mercuric reductase. Biochemistry 28:1194–1205
Miller S, Massey V, Ballou DP, Williams CH Jr, Distefano MD, Moore MJ, Walsh CT (1990) Use of a site-directed triple mutant to trap intermediates: demonstration that the flavin C(4a)-thiol adduct and reduced flavin are kinetically competent intermediates in mercuric reductase. Biochemistry 29:2831–2841
Miller S, Massey V, Williams CH Jr, Ballou DP, Walsh CT (1991) Communication between the actives sites in dimeric mercuric ion reductase: an alternating sites hypothesis for catalysis. Biochemistry 30:2600–2612
Mittl PRE, Schulz GE (1994) Structure of glutathione reductase from Escherichia coli at 1.86 Å resolution: comparison with the enzyme from human erythrocytes. Prot Science 3:799–809
Monaco HL (1997) Crystal structure of chicken riboflavin-binding protein. EMBO J 16:1475–1483
Moore MJ, Walsh CT (1989) Mutagenesis of the N- and C-terminal cysteine pairs of Tn501 mercuric ion reductase: consequences for bacterial detoxification of mercurials. Biochemistry 28:1183–1194
Moore MJ, Distefano MD, Zydowsky LD, Cummings RT, Walsh CT (1990) Organomercurial lyase and mercuric ion reductase: nature’s mercury detoxification catalysts. Acc Chem Res 23:301–308
Navascuès J, Pérez-Rontomé C, Gay M, Marcos M, Yang F, Walker FA, Desbois A, Abian J, Becana M (2012) Leghemoglobin green derivatives with nitrated hemes evidence production of highly reactive nitrogen species during aging of legume nodules. Proc Natl Acad Sci USA 109:2660–2665
Nishina Y, Kitagawa T, Shiga K, Horiike K, Matsumura Y, Watari H, Yamano T (1978) Resonance Raman spectra of riboflavin and its derivatives in the bound state with egg riboflavin binding proteins. J Biochem 84:925–932
Nishina Y, Kitagawa T, Shiga K, Watari H, Yamano T (1980) Resonance Raman study of flavoenzyme-inhibitor charge-transfer interactions. Old yellow enzyme-phenol complexes. J Biochem 87:831–839
Nishina Y, Shiga K, Tojo H, Miura R, Watari H, Yamano T (1981) Resonance Raman study of d-amino acid oxidase–inhibitor complexes. J Biochem 90:1515–1520
Nishina Y, Sato K, Shiga K, Fujii S, Kuroda K, Miura R (1992) Resonance Raman study on complexes of medium-chain acyl-CoA dehydrogenase. J Biochem 111:699–706
Nishina Y, Sato K, Shi R, Setoyama C, Miura R, Shiga K (2001) On the ligands in charge-transfer complexes of porcine kidney flavoenzyme d-amino acid oxidase in three redox states. A resonance Raman study. J Biochem 130:637–647
Orville AM, Lountos GT, Finnegan S, Gadda G, Prabhakar R (2009) Crystallographic, spectroscopic, and computational analysis of a flavin C4a–oxygen adduct in choline oxidase. Biochemistry 48:720–728
Parsonage D, Miller H, Ross RP, Claiborne A (1993) Purification and analysis of streptococcal NADH peroxidase expressed in Escherichia coli. J Biol Chem 268:3161–3167
Pawlukojć A, Natkaniec I, Bator G, Sobczyk L, Grech E, Nowicka-Scheibe J (2006) Low frequency internal modes of 1,2,4,5-tetramethylbenzene, tetramethylpyrazine and tetramethyl-1,4-benzoquinone. INS, Raman, infrared and theoretical DFT studies. Spectrochim Acta 63A:766–773
Picaud T, Desbois A (2002) Electrostatic control of the isoalloxazine environment in the two-electron reduced states of yeast glutathione reductase. J Biol Chem 277:31715–31721
Picaud T, Desbois A (2006) Interaction of glutathione reductase with heavy metals: the binding of Hg(II) or Cd(II) to the reduced enzyme affects both the redox dithiol pair and the flavin. Biochemistry 45:15829–15837
Poole LB, Claiborne A (1986) Interactions of pyridine nucleotides with redox forms of the flavin-containing NADH peroxidase from Streptococcus faecalis. J Biol Chem 261:14525–14533
Poole LB, Claiborne A (1989) The non-flavin redox center of the streptococcal NADH peroxidase. II. Evidence for a stabilized cysteine-sulfenic acid. J Biol Chem 264:12330–12338
Rieff B, Bauer S, Mathias G, Tavan P (2011) IR spectra of flavins in solution: DFT/MM description of redox effects. Femtosecond stimulated Raman spectroscopy of flavin after optical excitation. J Phys Chem B 115:2117–2123
Rinderle SJ, Booth JE, Williams JW (1983) Mercuric reductase from R-Plasmid NR1: characterization and mechanistic study. Biochemistry 22:869–876
Røhr AK, Hersleth HP, Andersson KK (2010) Tracking flavin conformations in protein crystal structures with Raman spectroscopy and QM/MM calculations. Angew Chem Int Ed 49:2324–2327
Rossy E, Champier L, Bersch B, Brutscher B, Blackledge M, Covès J (2004) Biophysical characterization of the MerP-like amino-terminal extension of the mercuric reductase from Ralstonia metallidurans CH34. J Biol Inorg Chem 9:49–58
Sahlman L, Lambeir AM, Lindskog S, Dunford HB (1984) The reaction between NADPH and mercuric reductase from Pseudomonas aeruginosa. J Biol Chem 259:12403–12408
Sahlman L, Lambeir AM, Lindskog S (1986) Rapid-scan stopped-flow studies of the pH dependence of the reaction between mercuric reductase and NADPH. Eur J Biochem 156:479–488
Sandström A, Lindskog S (1987) Rapid-scan stopped-flow studies of the flavoenzyme mercuric reductase during catalytic turnover. Eur J Biochem 164:243–249
Sayed A, Ghazy MA, Ferreira AJS, Setubal JC, Chambergo FS, Ouf A, Adel M, Dawe AS, Archer JAC, Bajic VB, Siam R, El-Dorry H (2014) A novel mercuric reductase from the unique deep brine environment of Atlantis II in the Red Sea. J Biol Chem 289:1675–1687
Schelvis JPM, Pun D, Goyal N, Sokolova O (2006) Resonance Raman spectra of the neutral and anionic radical semiquinones of flavin adenine dinucleotide in glucose oxidase revisited. J Raman Spectrosc 37:822–829
Schiering N, Kabsch W, Moore MJ, Distefano MD, Walsh CT, Pai EF (1991) Structure of the detoxification catalyst mercuric ion reductase from Bacillus sp. strain RC607. Nature 352:168–172
Schmidt J, Reinsch J, McFarland JT (1981) Mechanistic studies on fatty acyl-CoA dehydrogenase. J Biol Chem 256:11667–11670
Schmidt J, Coudron P, Thompson AW, Watters KL, McFarland JT (1983) Hydrogen bonding between flavin and protein: a resonance Raman study. Biochemistry 22:76–84
Schottel JL (1978) The mercuric and organomercurial detoxifying enzymes from a plasmid-bearing strain of Escherichia coli. J Biol Chem 253:4341–4349
Schreuder HA, Prick PAJ, Wierenga RK, Vriend G, Wilson KS, Hol WGJ, Drenth J (1989) Crystal structure of the p-hydroxybenzoate hydroxylase-substrate complex refined at 1.9 Å resolution. Analysis of the enzyme-substrate and enzyme-product complexes. J Mol Biol 208:679–696
Smith WW, Burnett RM, Darling GD, Ludwig ML (1977) Structure of the semiquinone form of flavodoxin from Clostridium MP. Extension of 1.8 Å resolution and some comparisons with the oxidized state. J Mol Biol 117:195–225
Stehle T, Ahmed SA, Claiborne A, Schulz GE (1991) Structure of NADH peroxidase from Streptococcus faecalis 10C1 refined at 2.16 Å resolution. J Mol Biol 221:1325–1344
Stehle T, Claiborne A, Schulz GE (1993) NADH binding site and catalysis of NADH peroxidase. Eur J Biochem 211:221–226
Stoll VS, Blanchard JS (1991) Chemical mechanism and rate-limiting steps in the reaction catalysed by Streptococcus faecalis NADH peroxidase. Biochemistry 30:942–948
Sugiyama T, Nisimoto Y, Mason HS, Loehr TM (1985) Flavins in NADPH-cytochrome P450 reductase: evidence for structural alteration of flavins in their one-electron-reduced semiquinone states from resonance Raman spectroscopy. Biochemistry 24:3012–3019
Tegoni M, Gervais M, Desbois A (1997) Resonance Raman study on the oxidized and anionic semiquinone forms of flavocytochrome b2 and l-lactate monooxygenase. Influence of the structure and environment of the isoalloxazine ring on the flavin function. Biochemistry 36:8932–8946
Unno M, Sano R, Masuda S, Ono T, Yamauchi S (2005) Light-Induced Structural Changes in the active site of the BLUF domain in AppA by Raman spectroscopy. J Phys Chem B 109:12620–12626
Unno M, Tsukiji Y, Kubota K, Masuda S (2012) N-terminal truncation does not affect the location of a conserved tryptophan in the BLUF domain of AppA from Rhodobacter sphaeroides. J Phys Chem B 116:8974–8980
Visser AJWG, Vervoort J, O’Kane DJ, Lee J, Carreira LA (1983) Raman spectra of flavin bound in flavodoxins and in other flavoproteins. Evidence for structural variations in the flavin-binding region. Eur J Biochem 131:639–645
Vrielink A, Lloyd LF, Blow DM (1991) Crystal structure of cholesterol oxidase from Brevobacterium sterolicum refined at 1.8 Å resolution. J Mol Biol 219:533–554
Watt W, Tulinski A, Swenson RP, Watenpaugh KD (1991) Comparison of the crystal structures of a flavodoxin in its three oxidation states at cryogenic temperatures. J Mol Biol 218:195–208
Weigel A, Dobryakov A, Klaumünzer B, Sajadi M, Saalfrank P, Ernsting NP (2011) Femtosecond stimulated Raman spectroscopy of flavin after optical excitation. J Phys Chem B 115:3656–3680
Wille G, Ritter M, Friedemann R, Mäntele W, Hübner G (2003) Redox-triggered FTIR difference spectra of FAD in aqueous solution and bound to flavoproteins. Biochemistry 42:14814–14821
Williams CH Jr (1992) Lipoamide dehydrogenase, glutathione reductase, thioredoxin reductase, and mercuric ion reductase—a family of flavoenzyme transhydrogenases. In: Müller F (ed) Chemistry and biochemistry of flavoenzymes, vol 3. CRC Press, Boca Raton, pp 121–211
Williamson G, Engel PC, Nishina Y, Shiga KA (1982) Resonance Raman study on the nature of charge-transfer interactions in butyryl CoA dehydrogenase. FEBS Lett 138:29–32
Wohlfahrt G, Witt S, Hendle J, Schomburg D, Kalisz HM, Hecht HJ (1999) 1.8 and 1.9 Å resolution structures of the Penicillium amagasakiense and Aspergillus niger glucose oxidases as a basis for modeling substrate complexes. Acta Crystallogr D 55:969–977
Wolf MMN, Schumann C, Gross R, Domratcheva T, Diller R (2008) Ultrafast infrared spectroscopy of riboflavin: dynamics, electronic structure, and vibrational mode analysis. J Phys Chem B 112:13424–13432
Xia ZX, Mathews FS (1990) Molecular structure of flavocytochrome b2 at 2.4 Å resolution. J Mol Biol 212:837–863
Yang KY, Swenson RP (2007) Nonresonance Raman study of the flavin cofactor and its interactions in the methylotrophic bacterium W3A1 electron-transfer flavoprotein. Biochemistry 46:2298–2305
Yeh JI, Claiborne A, Hol WGJ (1996) Structure of the native cysteine-sulfenic acid redox center of enterococcal NADH peroxidase refined at 2.8 Å resolution. Biochemistry 35:9951–9957
Zheng Y (2002) Raman spectroscopic studies of flavoprotein chemistry. PhD thesis, Case Western Reserve University, Cleveland, OH
Zheng Y, Dong J, Palfey BA, Carey PR (1999) Using Raman spectroscopy to monitor the solvent-exposed and “buried” forms of flavin in p-hydroxybenzoate hydroxylase. Biochemistry 38:16727–16732
Ziegler GA, Vonrhein C, Hanukoglu I, Schulz GE (1999) The structure of adrenodoxin reductase of mitiochondrial P450 systems: electron transfer for steroid biosynthesis. J Mol Biol 289:981–990
Acknowledgements
We are grateful to Dr. J. Covès, for providing the E. coli strain CM037 harbouring the Tn4378 transposon which contains a mer operon of R. metallidurans CH34, and to Drs. F. André and P. Dorlet for the helpful discussions. This work was supported in part by grants from the Commissariat à l’Energie Atomique, and the Centre National de la Recherche Scientifique.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
249_2017_1245_MOESM1_ESM.docx
Five figures (RR spectra of MerA reduced with either DTT or dithionite; comparison of the RDs of Npx, MerA, and GR under the Eox state; comparison of the RDs of Npx, MerA, and GR under the EH2 states generated by NAD(P)H; RDs of GR under the Eox and EH2 states; RDs of MerA under the EH2 states generated by either DTT or dithionite) (DOCX 102 kb)
Rights and permissions
About this article
Cite this article
Keirsse-Haquin, J., Picaud, T., Bordes, L. et al. Modulation of the flavin–protein interactions in NADH peroxidase and mercuric ion reductase: a resonance Raman study. Eur Biophys J 47, 205–223 (2018). https://doi.org/10.1007/s00249-017-1245-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-017-1245-3