Abstract
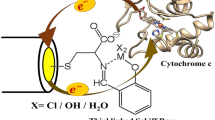
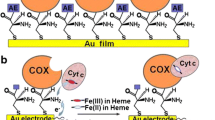
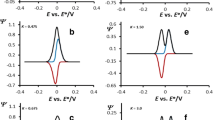
Cytochrome c 552 (Cyt-c 552) and its redox partner ba 3 -oxidase from Thermus thermophilus possess structural differences compared with Horse heart cytochrome c (cyt-c)/cytochrome c oxidase (CcO) system, where the recognition between partners and the electron transfer (ET) process is initiated via electrostatic interactions. We demonstrated in a previous study by surface-enhanced resonance Raman (SERR) spectroscopy that roughened silver electrodes coated with uncharged mixed self-assembled monolayers HS–(CH2) n –CH3/HS–(CH2) n + 1–OH 50/50, n = 5, 10 or 15, was a good model to mimic the Cyt-c 552 redox partner. All the adsorbed molecules are well oriented on such biomimetic electrodes and transfer one electron during the redox process. The present work focuses on the kinetic part of the heterogeneous ET process of Cyt-c 552 adsorbed onto electrodes coated with such mixed SAMs of different alkyl chain length. For that purpose, two complementary methods were combined. Firstly cyclic voltammetry shows that the ET between the adsorbed Cyt-c 552 and the biomimetic electrode is direct and reversible. Furthermore, it allows the estimation of both the density surface coverage of adsorbed Cyt-c 552 and the kinetic constants values. Secondly, time-resolved SERR (TR-SERR) spectroscopy showed that the ET process occurs without conformational change of the Cyt-c 552 heme group and allows the determination of kinetic constants. Results show that the kinetic constant values obtained by TR-SERR spectroscopy could be compared to those obtained from cyclic voltammetry. They are estimated at 200, 150 and 40 s−1 for the ET of Cyt-c 552 adsorbed onto electrodes coated with mixed SAMs HS–(CH2) n –CH3/HS–(CH2) n + 1–OH 50/50, n = 5, 10 or 15, respectively.






Similar content being viewed by others
References
Avila A, Gregory BW, Niki K, Cotton TM (2000) An electrochemical approach to investigate gated electron transfer using a physiological model system: cytochrome c immobilized on carboxylic acid-terminated alkanethiol self-assembled monolayers on gold electrodes. J Phys Chem B 104:2759–2766
Bard AJ, Faulkner LR (2001) Electrochemical methods: fundamentals and applications. Wiley, New York
Battistuzzi G, Borsari M, Cowan JA, Ranieri A, Sola M (2002) Control of cytochrome c redox potential: axial ligation and protein environment effect. J Am Chem Soc 124:5315–5324
Bernad S, Soulimane T, Lecomte S (2004) Redox and conformational equilibria of cytochrome c552 from Thermus thermipohilus adsorbed on chemically modified silver electrode probed by SERR spectroscopy. J Raman Spectrosc 35:47–54
Bernad S, Soulimane T, Lecomte S (2006) Characterization and redox properties of cytochrome c552 from Thermus thermophilus adsorbed on different self-assembled thiol monolayers, used to model the chemical environment of the redox partner. Biopolymers 81:407–418
Clark RA, Bowden EF (1997) Voltametric peak broadening for cytochrome c/alkanethiolate monolayer structures: dispersion of formal potentials. Langmuir 13:559–565
Cotton TM, Schultz SG, Van Duyne RP (1980) Surface-enhanced resonance Raman scattering from cytochrome c and myoglobin adsorbed on a silver electrode. J Am Chem Soc 102:7960–7962
Döpner S, Hildebrandt P, Rosell FI, Mauk AG, von Walter M, Buse G, Soulimane T (1999) The structural and functional role of lysine residues in the binding domain of cytochrome c in the electron transfer to cytochrome c oxidase. Eur J Biochem 261:379–391
El Kasmi A, Wallace JM, Bowden EF, Binet SM, Linderman RJ (1998) Controlling interfacial electron transfer kinetics of cytochrome c with mixed self-assembled monolayers. J Am Chem Soc 120:225–226
Feng ZQ, Imabayashi S, T. K, Niki K (1995) Electroreflectance spectroscopic study of the electron transfer rate of cytochrome c electrostatically immobilized on the o-carboxyl alkanethiol monolayer modified gold electrode. J Electroanal Chem 394:149–154
Feng ZQ, Imabayashi S, Kakiushi T, Niki K (1997) Long-range electron-transfer reaction rates to cytochrome across long- and short- chain alkanethiol self-assembled monolayers: electroreflectance studies. J Chem Soc Faraday Trans 93:1367–1370
Hildebrandt P, Macor KA, Czernuszewicz RS (1988) Novel cylindrical rotating electrode for anaerobic surface-enhanced Raman spectroscopy. J Raman Spectrosc 19:65–69
Hildebrandt P, Murgida DH (2002) Electron transfer dynamics of cytochrome c bound to self-assembled monolayers on silver electrodes. Bioelectrochemistry 55:139–143
Hildebrandt P, Stockburger M (1989a) Cytochrome c at charged interfaces 1: conformational and redox equilibria at the electrode/electrolyte interface probed by surface-enhanced resonance Raman spectroscopy. Biochemistry 28:6710–6721
Hildebrandt P, Stockburger M (1989b) Cytochrome c at charged interfaces 2: complexes with negatively charged macromolecular systems studied by resonance Raman spectroscopy. Biochemistry 28:6722–6728
Hon-Nami K, Oshim T (1977) Purification and some properties of cytochrome c552 from an extreme thermophile, Thermus thermophilus. J Biochem 82:769–776
Hu S, Morris IK, Singh JP, Smith KM, Spiro TG (1993) Complete assignment of cytochrome c resonance Raman spectra via enzymatic reconstitution with isotopically labeled hemes. J Am Chem Soc 115:12446–12458
Jeuken LJC (2003) Conformational reorganisation in interfacial protein electron transfer. Biochim Biophys Acta 1604:67–76
Laviron E (1979) General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J Electroanal Chem 101:19–28
Lecomte S, Hildebrandt P, Soulimane T (1999) Dynamics of the heterogeneous electron-transfer reaction of cytochrome c 552 from Thermus thermophilus. A time-resolved surface-enhanced resonance Raman spectroscopic study. J Phys Chem B 103:10053–10064
Lecomte S, Wackerbarth H, Hildebrandt P, Soulimane T, Buse G (1998a) Potential-dependent surface-enhanced resonance Raman spectroscopy of cytochrome c 552 from Thermus thermophilus. J Raman Spectrosc 29:687–692
Lecomte S, Wackerbarth H, Soulimane T, Buse G, Hildebrandt P (1998b) Time-resolved surface-enhanced resonance Raman spectroscopy for stuying electron -transfer dynamics of heme proteins. J Am Chem Soc 120:7381–7382
Leopold MC, Bowden EF (2002) Influence of gold substrate topography on the voltammetry of cytochrome c adsorbed on carboxylic acid terminated self-assembled monolayers. Langmuir 18:2239–2245
Marcus RA, Sutin N (1985) Electron transfers in chemistry and biology. Biochim Biophys Acta 811:265–322
Murgida DH, Hildebrandt P (2001) Proton-coupled electron transfer of cytochrome c. J Am Chem Soc 123:4062–4068
Murgida DH, Hildebrandt P (2004) Electron-transfer processes of cytochrome c at interfaces: new insights by surface-enhanced resonance Raman spectroscopy. Acc Chem Res 37:854–861
Scott RA, Mauk AG (1995) Cytochrome c: a multidisciplinary approach. University Science Books, Sausalito
Song S, Clark RA, Bowden EF (1993) Characterization of cytochrome c/alkanethiolate structures prepared by self-assembly on gold. J Phys Chem 97:6564–6572
Soulimane T, Buse G, Bourenkov GP, Bartunik HD, Huber R, Than ME (2000) Structure and mechanism of the aberrant ba 3-cytochrome c oxydases from Thermus thermophilus. EMBO Journal 19:1766–1776
Soulimane T, Von Walter M, Hof P, Than ME, Huber R, Buse G (1997) Cytochrome c 552 from Thermus thermophilus: a functional and crystallographic investigation. Biochemical and Biophysical Research Commun 237:572–576
Than ME, Hof P, Huber R, Bourenkov GP, Bartunik HD, Buse G, Soulimane T (1997) Thermus thermophilus cytochrome c 552: a new highly thermostable cytochrome c structure obtained by MAD phasing. J Mol Biol 271:629–644
Than ME, Soulimane T (2001) Ba 3-Cytochrome c oxydase from Thermus thermophilus. In: Messerschmidt A, Huber R, Poulos T, Wieghardt K (eds) Handbook of metalloproteins, Chichester, pp 363–378
Wackerbarth H, Klar U, Günther W, Hildebrandt P (1999) Novel time-resolved surface-enhanced (resonance) Raman spectroscopic technique for studying the dynamics of interfacial processes: application to the electron transfer reaction of cytochrome c at silver electrode. Appl Spectrosc 53:283–291
Wei J, Liu AR, Yamamoto H, He Y, Waldeck DH (2002) Direct wiring of cytochrme c’s heme unit to an electrode: electrochemical studies. J Am Chem Soc 124:9591–9599
Wei JJ, Liu H, Niki K, Margoliash E, Waldeck DH (2004) Probing electron tunneling pathways: electrochemical study of rat heart cytochrome c and its mutant on pyridine-terminated SAMs. J Phys Chem B 108:16912–16917
Yue H, Khoshtariya D, Waldeck DH, Grochol J, Hildebrandt P, Murgida DH (2006) On the electron transfer mechanism between cytochrome c and metal electrodes: evidence for dynamics control at short distances. J Phys Chem B 110:19906–19913
Acknowledgment
The authors thank Dr Tewfik Soulimane for the gift of Cyt-c 552.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bernad, S., Leygue, N., Korri-Youssoufi, H. et al. Kinetics of the electron transfer reaction of Cytochrome c 552 adsorbed on biomimetic electrode studied by time-resolved surface-enhanced resonance Raman spectroscopy and electrochemistry. Eur Biophys J 36, 1039–1048 (2007). https://doi.org/10.1007/s00249-007-0173-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-007-0173-z