Abstract
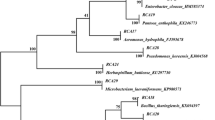
During a survey of endophytic diazotrophic bacteria associated with different rice varieties in Tamilnadu, some “endophytes” were obtained. Thirteen bacterial isolates from surface-sterilized roots and shoots were obtained in pure culture, which produced indole acetic acid (IAA) and reduced acetylene to ethylene. Polymerase chain reaction (PCR) amplification confirmed the presence of nif-H gene in all the isolates. Morphological, biochemical, and molecular characteristics indicated that all of them belonged to the genus Burkholderia One of them, MGK3, was consistently more active in reducing acetylene, and 16S rDNA sequences of isolate MGK3 confirmed its identification as Burkholderia vietnamiensis. Colonization of rice root was confirmed by strain MGK3 marked with gusA gene. The inoculated roots showed a blue color, which was most intense at the points of lateral root emergence and at the root tip. Transverse sections of roots, 15 days after inoculation, revealed beta-glucuronidase (GUS) activity within many of the cortical intercellular spaces next to the stele and within the aerenchyma. Nitrogen fixation was quantified by using 15N isotope dilution method with two different cultivars grown in pot and field experiments. Higher nitrogen fixation was observed in variety Ponni than in ADT-43, where nearly 42% (field) and 40% (pot) of the nitrogen was derived from the atmosphere (% Ndfa). Isolate MGK3 was used to inoculate rice seedlings in a comparison with four other diazotrophs, viz., Gluconacetobacter diazotrophicus LMG7603, Herbaspirillum seropedicae LMG6513, Azospirillum lipoferum 4B LMG4348, and B. vietnamiensis LMG10929. They were used to conduct two pot and four field inoculation experiments. MGK3 alone, and combined with other diazotrophs, performed best under both pot and field conditions: combined inoculation produced yield increases between 9.5 and 23.6%, while MGK3 alone increased yield by 5.6 to 12.16% over the uninoculated control treatment.


Similar content being viewed by others
References
Andrews, MA, James, EKB, Cummings, SPA, Zavalin, AAC, Vinogradova, LVC, McKenzie, BAD (2003) Use of nitrogen fixing bacteria inoculants as a substitute for nitrogen fertiliser for dry land graminaceous crops: progress made, mechanisms of action and future potential. Symbiosis 35: 209–229
Balandreau, J, Viallard, V, Cournoyer, B, Coenye, T, Laevens, S, Vandamme, P (2001) Burkholderia cepacia genomovar III is a common plant-associated bacterium. Appl Environ Microbiol 67: 982–985
Baldani, JI, Baldani, VLD, Seldin, L, Dobereiner, J (1986) Characterisation of Herbaspirillum seropedicae gen. nov. sp. nov., a root associated nitrogen fixing bacterium. Int J Syst Bacteriol 36: 86–93
Baldani, VLD, Baldani, JI, Dobereiner, J (2000) Inoculation of rice plants with the endophytic diazotrophs Herbaspirillum seropedicae and Burkholderia spp. Biol Fertil Soils 30: 485–491
Baldani, JI, Pot, B, Kirchhof, G, Falsen, E, Baldani, VLD, Olivares, FL, Hoste, B, Kersters, K, Hartmann, A, Gillis, M, Döbereiner, J (1996) Emended description of Herbaspirillum; inclusion of [Pseudomonas] rubrisubalbicans, a mild plant pathogen, as Herbaspirillum rubrisubalbicans comb. nov.; and classification of a group of clinical isolates (EF group 1) as Herbaspirillum species 3. Int J Syst Bacteriol 46: 802–810
Bally, I, Thomas-Bauzon, D, Heulin, T, Balandreau, J, Richard, C, De Ley, J (1983) Determination of the most frequent N2-fixing bacteria in a rice rhizosphere. Can J Microbiol 29: 881–887
Bilal, R, Malik, KA (1987) Isolation and identification of an N2-fixing zoogloea-forming bacterium from kallar grass histoplane. J Appl Bacteriol 62: 289–294
Bilal, R, Rasul, G, Qureshi, JA, Malik, KA (1990) Characterisation of Azospirillum and related diazotrophs associated with roots of plants growing in saline soils. World J Microbiol Biotechnol 6: 46–52
Boddey, R, Dobereiner, J (1988) Nitrogen fixation associated with grasses and cereals: recent results and perspectives for future research. Plant Soil 108: 53–65
Boddey, RM, Dobereiner, J (1995) Nitrogen fixation associated with grasses and cereals; recent progress and perspectives for the future. Fertil Res 42: 241–250
Boddey, RM, Polidoro, JC, Resende, AS, Alves, BJR, Urquiaga, S (2001) Use of the 15N natural abundance technique for the quantification of the contribution of N2 fixation to sugarcane and other grasses. Aust J Plant Physiol 28: 889–895
Burbage, DA, Sasser, M (1982) A medium selective for Pseudomonas cepacia. Phytopathol Abstracts 72: 706
Caballero-Mellado, J, Martínez-Aguilar, L, Paredes-Valdez, G, Estrada-de los Santos, P (2004) Burkholderia unamae sp. nov., an N2-fixing rhizospheric and endophytic species. Int J Syst Bacteriol 54: 1165–1172
Charyulu, PBBN, Fourcassie, F, Barbouche, AK, Rondro, Harisoa, L, Omar AMN, Weinhard, P, Marie, R, Balandreau, J (1985) Field inoculation of rice using in vitro selected bacterial and plant genotypes. In: Klingmuller W (Eds.) Azospirillum III. Genetics Physiology Ecology, Springer-Verlag Publications, pp 163–179
Chen, WM, de Fario, SM, Straaliotto, R, Pitard, RM, Simoes-Araujo, JL, Chou, JH, Chou, YJ, Barrios, E, Prescott, AR, Elliott, GN, Sprent, JI, Young, JPW, James, EK (2005a) Proof that Burkholderia forms effective symbioses with legumes: a study of novel mimosa-nodulating strains from South America. Appl Environ Microbiol 71: 7461–7471
Chen, WM, James, EK, Chou, JH, Sheu, SY, Yang, SZ, Sprent, JI (2005b) Beta-rhizobia from Mimosa pigra, a newly discovered invasive plant in Taiwan. New Phytol 168: 661–675
Christiansen-Weniger, C (1997) Ammonium-excreting Azospirillum brasilense C3: gusA inhabiting induced tumors along stem and roots of rice. Soil Biol Biochem 29: 943–950
Di cello, F, Bevivino, A, Chiarini, L, Fani, R, Paffetti, D, Tabachioni, S, Dalmastri, C (1997) Biodiversity of a Burkholderia cepacia population isolated from the maize rhizosphere at different growth stages. Appl Environ Microbiol 63: 4485–4493
Döbereiner, J, Day, JM (1976) Associative symbiosis in tropical grasses. Characterization of microorganisms and dinitrogen fixing sites. In: Newton, WE, Nyman, CJ (Eds.) Proceedings of the first international symposium on nitrogen fixation, Washington State University Press, Pullman, 2, pp 518–538
Dobereiner, J, Reis, V, Paula, M, Olivares, F (1993) Endophytic diazotrophs in sugar cane, cereals, and tuber plants. In: Palacios, R, Mora, J, Newton, WE (Eds.) ‘New Horizons in Nitrogen Fixation’, Kluwer Academic Publishers, Dordrecht, pp 671–679
Elbeltagy, A, Nishioka, K, Sato, T, Suzuki, H, Ye, B, Hamada, T, Isawa, T, Mitsui, H, Minamisawa, K (2001) Endophytic colonization and in planta nitrogen fixation by a Herbaspirillum sp. isolated from wild rice species. Appl Environ Microbiol 67: 5285–5293
Estrada-de-los-Santos, P, Bustillo-Cristalles, R, Caballero-Mellado, J (2001) Burkholderia, a genus rich in plant-associated nitrogen fixers with wide environmental and geographical distribution. Appl Environ Microbiol 67: 2790–2798
Estrada, P, Mavingui, P, Cournoyer, B, Fontaine, F, Balndreau, J, Cabellero-Mellado, J (2002) A N2-fixing endophytic Burkholderia sp. associated with maize plants cultivated in Mexico. Can J Microbiol 48: 285–294
Fried, M, Middleboe, V (1977) Measurement of amount of nitrogen fixed by a legume crop. Plant Soil 40: 713–715
Gillis, M, Kersters, K, Hoste, B, Janssens, D, Kropenstedt, RM, Stephen, MP, Teixeira, KRS, Dobereiner, J, De Ley, J (1989) Acetobacter diazotrophicus sp. nov., a nitrogen-fixing acetic acid bacterium associated with sugarcane. Int J Syst Bacteriol 39: 361–364
Gillis, M, Tran Van, V, Bardin, R, Goor, M, Hebar, P, Willems, A, Segers, P, Kersters, K, Heulin, T, Fernandez, MP (1995) Polyphasic taxonomy in the genus Burkholderia leading to an emended description of the genus Burkholderia and transposition of Burkholderia vietnamiensis sp. nov. for N2-fixing isolates from rice in Vietnam. Int J Syst Bacteriol 45: 274–289
Goris, J, De Vos, P, Caballero-Mellado, J, Park, JH, Falsen, E, James M, Tiedje, Vandamme, P (2004) Classification of the PCB- and biphenyl-degrading strain LB400 and relatives as Burkholderia xenovorans sp. nov. Int J Syst Evol Microbiol 54: 1677–1681
Govindarajan, M, Balandreau, J, Muthukumarasamy, R, Revathi, G, Lakshminarasimhan, C (2006) Improved yield of micropropagated sugarcane following inoculation by endophytic Burkholderia vietnamiensis. Plant Soil 280: 239–252
Yang, H-C, Im, W-T, Kim, KK, An, D-S, Lee, S-T (2006) Burkholderia terrae sp. nov., isolated from a forest soil. Int J Syst Evol Microbiol 56: 453–457
Heulin, T, Rahman, M, Omar, AMN, Rafidison, Z, Pierrat, JC, Balandreau, J (1989) Experimental and mathematical procedures for comparing efficiencies of rhizosphere N2 fixing bacteria. J Microbiol Methods 9: 163–173
Hiraishi, A (1992) Direct automated sequencing of 16S rDNA amplified by polymerase chain reaction from bacterial cultures without DNA purification. Lett Appl Microbiol 15: 210–213
Humphries, EC (1956) Mineral components and ash analysis. In: Peach, K, Tracged, MV (Eds.) Modern Methods of Plant Analysis, Springer-Verlag, Berlin. pp 468–502
Hurek, T, Reinhold-Hurek, B, van Montague, M, Kellenberger, E (1994) Root colonization and systemic spreading of Azoarcus sp. strain BH72 in grasses. J Bacteriol 176: 1913–1923
Jacoud, C, Job, D, Wadoux, P, Bally, R (1999) Initiation of root growth stimulation by Azospirillum lipoferum CRT1 during maize seed germination. Can J Microbiol 45: 339–342
Jagnow, GC (1983) Nitrogenase (C2 H2) activity in non-cultivated and cereal plants; influence of nitrogen fertilizer on population and activity of nitrogen fixing bacteria. Z Pflanzenernaehr Bodenkd 146: 217–227
James, EK, Olivares, FL (1998) Infection and colonization of sugar cane and other graminaceous plants by endophytic diazotrophs. Crit Rev Plant Sci 17: 77–119
James, EK, Gyaneshwar, P, Mathan, N, Barraquio, WL, Reddy, PM, Iannetta, PP, Olivares, FL, Ladha, JK (2002) Infection and colonization of rice seedlings by the plant growth-promoting bacterium Herbaspirillum seropedicae Z67. Mol Plant Microb Interact 15: 894–906
Jukes, TH, Cantor, CR (1969) Evolution of protein molecules. In: Munro, HN (Eds.) Mammalian Protein Metabolism, Academic Press, New York, III, pp 21–132
Kennedy, IR, Tchan, YT (1992) Biological nitrogen fixation in non-leguminous field crops: recent advances. Plant Soil 141: 93–118
Kirchhof, G, Reis, VM, Baldani, JI, Eckert, B, Dobereiner, J, Hartmann, A (1997) Occurence, physiological and molecular analysis of endophytic diazotrophic bacteria in gramineous energy plants. Plant Soil 194: 45–55
Kirchhof, G, Baldani, JI, Reis, VM, Hartmann, A (1998) Molecular assay to identify Acetobacter diazotrophicus and detect its occurrence in plant tissues. Can J Microbiol 44: 12–19
Kumar, S, Tamura, K, Jakobsen, IB, Nei, M (2001) MEGA2: Molecular Evolutionary Genetics Analysis Software. Bioinformatics 17: 1244–1245
Kwon, SW, Kim JS, Park, IC, Yoon, SH, Park, DH, Lim, CK, Go, SJ (2003) Pseudomonas koreensis sp. nov., Pseudomonas umsongensis sp. nov. and Pseudomonas jinjuensis sp. nov., novel species from farm soils in Korea. Int J Syst Evol Microbiol 53: 21–27
Ladha, JK, Triol, AC, Daroy, LG, Caldo, G, Ventura, W, Watanabe, I (1986) Plant associated N2 fixation (C2H2) reduction by five rice varieties, and relationship with plant growth characters as affected by straw incorporation. Soil Sci Plant Nutr 32: 91–106
Mascarua-Esparza, MA, Villa-Gonzallez, R, Caballero-Mellado, J (1988) Acetylene reduction and indolacetic acid production by Azospirillum isolates from Cactaceous plants. Plant Soil 106: 91–95
Muthukumarasamy, R, Revathi, G, Lakshminarasimhan, C (1999) Diazotrophic associations in sugarcane cultivation in South India. Trop Agric (Trinidad) 76: 171–178
Oliveira, ALM, Urquiaga, S, Döbereiner, J, Baldani, JI (2002) The effect of inoculating endophytic N2-fixing bacteria on micropropagated sugarcane plants. Plant Soil 242: 205–215
Parke, JL, Gurian-Sherman, D (2001) Diversity of the Burkholderia cepacia complex and implications for risk assessment of biological control strains. Annu Rev Phytopathol 39: 225–258
Rasul, G, Mirza, MS, Latif, F, Malik, KA (1998) Identification of plant growth hormones produced by bacterial isolates from rice, wheat and kallar grass. In: Malik, KA, Mirza, MS, Ladha, JK (Eds.) Nitrogen Fixation with Non-Legumes, Kluwer Academic Publishers, Netherlands
Reinhold-Hurek, BR, Hurek, T, Gillis, M, Hoste, B, Vancanneyt, M, Kersters, K, De Ley, J (1993) Azoarcus gen. nov. Nitrogen-fixing proteobacteria associated with roots of kallar grass (Leptochoa fusca (L.) Kunth) and description of two species, Azoarcus indigens sp. nov. and Azoarcus communis sp. nov. Int J Syst Bacteriol 43: 574–584
Reis, VM, Olivares, FI, Dobereiner, J (1994) Improved methodology for isolation of Acetobacter diazotrophicus and confirmation of its endophytic habitat. World J Microbial Biotechnol 10: 101–104
Reis, VM, Estrada-de los Santos, P, Tenorio-Salgado, S, Vogel, J, Stoffels M, Guyon, S, Mavingui, P, Baldani, VLD, Schmid, M, Baldani, JI, Balandreau, J, Hartmann, A, Caballero-Mellado, J (2004) Burkholderia tropica sp. nov., a novel nitrogen-fixing, plant-associated bacterium. Int J Syst Evol Microbiol 54: 2155–2162
Saitou, N, Nei, M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4: 406–425
Sessitsch, Coenye, T, Sturz, AV, Vandamme, P, Ait Barka, E, Salles, JF, Van Elsas, JD, Faure, D, Reiter, B, Glick, BR, Wang-Pruski, G, Nowak, J (2005) Burkholderia phytofirmans sp. nov., a novel plant-associated bacterium with plant-beneficial properties. Int J Syst Evol Microbiol 55: 1187–1192
Sevilla, M, de Olivares, A, Baldani, JI, Kennedy, C (1998) Contribution of the bacterial endophyte Acetobacter diazotrophicus to sugarcane nutrition. Symbiosis 25: 181–191
Sevilla, M, Kennedy, C (2000) Colonisation of rice and other cereals by Acetobacter diazotrophicus, an endophyte of sugarcane. In: Ladha, JK, Reddy, PM (Eds.) The Quest for Nitrogen Fixation, IRRI Press, Manila, pp 151–165
Simon, R, Priefer, U, Puhler, A (1983) A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis of Gram negative bacteria. Bio/Technology 1: 784–791
Somasekaran, P, Huben, HJ (1994) Handbook for Rhizobia. Springer-Verlag, pp 340
Thomas-Bauzon, D, Weinhard, P, Villecourt, P, Balandreau, J (1982) The spermosphere model. Its use in growing counting and isolating N2-fixing bacteria from the rhizosphere of rice. Can J Microbiol 28: 922–928
Tien, TM, Gaskins, MH, Hubbell, DH (1979) Plant growth substances produced by Azospirillum brasiliense and their effect on growth of Pearl millet (Pennisetum americanum L.). Appl Environ Microbiol 37: 1016–1024
Tran Van, V, Berge, O, Balandreau, J, Ngo Kê, S, Heulin, T (1996) Isolement et activité nitrogénasique de Burkholderia vietnamiensis, bactérie fixatrice d’azote associée au riz (Oryza sativa L) cultivé sur un sol sulfaté acide du Viêt-nam. Agronomie 16: 479–491
Tran Van, V, Berge, O, Ngo Ke, S, Balandreau, J, Heulin, T (2000) Repeated beneficial effects of rice inoculation with a strain of Burkholderia vietnamiensis on early and late yield components in low fertility sulphate acid soils of Vietnam. Plant Soil 218: 273–284
Ueda, T, Suga, Y, Yahiro, N, Matsuguchi, T (1995) Remarkable N2-fixing bacterial diversity detected in rice roots by molecular evolutionary analysis of nif-H gene sequence. J Bacteriol 177: 1414–1417
Vandamme, P, Goris, J, Chen, WM, de Vos P, Willems, A (2002) Burkholderia tuberum sp. nov. and Burkholderia phymatum sp. nov., nodulate the roots of tropical legumes. Syst Appl Microbiol 25: 507–512
Watanabe, I, Barraquio, WI, de Guzman, MR, Cabera, DA (1979) Nitrogen fixing (C2 H2 reduction) activity and population of aerobic heterotrophic nitrogen fixing bacteria associated with wetland rice. Appl Environ Microbiol 37: 813–819
Wilson, KJ, Sessittsch, A, Corbo, J, Giller, KEA, Akkermans, DL, Jefferson, RA (1995) β-glucuronidase (Gus) transposons for ecological for ecological and genetic studies of rhizobia and other gram negative bacteria. Microbiology 141: 1691–1705
Yamada, Y, Hoshino, K, Ishikawa, T (1997) The phylogeny of acetic acid bacteria based on the partial sequences of 16S ribosomal RNA: the elevation of the subgenus Gluconoacetobacter to the generic level. Biosci Biotechnol Biochem 61: 1244–1251
Yanni, YG, Rizk, RY, Abd El-Fattah, FK, et al. (2001) The beneficial plant growth-promoting association of Rhizobium leguminosarum bv. trifolii with rice roots. Aust J Plant Physiol 28: 1–26
Acknowledgments
This work is part of the Ph.D. thesis of first author. Thanks are due to Prof. P. Vandamme LMG-BCCM, University of Gent, Belgium for providing B. vietnamiensis and B. cepacia reference cultures. For reprints, preprints, unpublished data, practical assistance, and helpful discussions, we thank our friends and colleagues in throughout the world.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Govindarajan, M., Balandreau, J., Kwon, SW. et al. Effects of the Inoculation of Burkholderia vietnamensis and Related Endophytic Diazotrophic Bacteria on Grain Yield of Rice. Microb Ecol 55, 21–37 (2008). https://doi.org/10.1007/s00248-007-9247-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-007-9247-9