Abstract
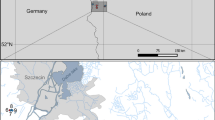
Wetlands are major sources of carbon dioxide, methane, and other greenhouse gases released during microbial degradation. Despite the fact that decomposition is mainly driven by bacteria and fungi, little is known about the taxonomic diversity of bacterial communities in wetlands, particularly Sphagnum bogs. To explore bacterial community composition, 24 bogs in Vermont and Massachusetts were censused for bacterial diversity at the surface (oxic) and 1 m (anoxic) regions. Bacterial diversity was characterized by a terminal restriction fragment length (T-RFLP) fingerprinting technique and a cloning strategy that targeted the 16S rRNA gene. T-RFLP analysis revealed a high level of diversity, and a canonical correspondence analysis demonstrated marked similarity among bogs, but consistent differences between surface and subsurface assemblages. 16S rDNA sequences derived from one of the sites showed high numbers of clones belonging to the Deltaproteobacteria group. Several other phyla were represented, as well as two Candidate Division-level taxonomic groups. These data suggest that bog microbial communities are complex, possibly stratified, and similar among multiple sites.
Similar content being viewed by others
References
CB Blackwood, TL Marsh, S-H Kim and EA Paul, Terminal restriction fragment length polymorphism data analysis for quantitative comparison of microbial communities. Appl Environ Microbiol 69 (2003) 926-932
G Braker, HL Ayala del Río, AH Devol, A Fesefeldt and JM Tiedje, Community structure of denitrifiers, bacteria and Archaea along redox gradients in Pacific Northwest marine sediments by terminal restriction fragment length polymorphism analysis of amplified nitrite reductase (nirS) and 16S rRNA genes. Appl Environ Microbiol 67 (2001) 1893-1901
JE Brofft, JV McArthur and LJ Shimkets, Recovery of novel bacterial diversity from a forested wetland impacted by reject coal. Environ Microbiol 4 (2002) 764-769
DH Buckley and TM Schmidt, Diversity and dynamics of microbial communities in soils from agro-ecosystems. Environ Microbiol 5 (2003) 441-452
HF Castro, NH Williams and A Ogram, Phylogeny of sulfate-reducing bacteria. FEMS Microbiol Ecol 31 (2000) 1-9
DE Cummings, AW March, B Bostick, S Spring, F Caccavo Jr, S Fendorf and RF Rosenzweig, Evidence for microbial Fe(III) reduction in anoxic, mining-impacted lake sediments (Lake Coeur d’Alene, Idaho). Appl Environ Microbiol 66 (2000) 154-162
SN Dedysh, NS Panikov, W Liesack, R Großkopf, J Zhou and JM Tiedje, Isolation of acidophilc methane-oxidizing bacteria from Northern peat wetlands. Science 282 (1998) 281-284
A Dhillon, A Teske, J Dillon, DA Stahl and ML Sogin, Molecular characterization of sulfate-reducing bacteria in the Guaymas basin. Appl Environ Microbiol 69 (2003) 2765-2772
JM Dunbar, LO Ticknor and CR Kuske, Assessment of microbial diversity in four Southwestern United States soils by 16S rRNA gene terminal restriction fragment analysis. Appl Environ Microbiol 66 (2000) 2943-2950
B Duval, Methane production and release from two New England peatlands. Int Microbiol 3 (2000) 89-95
AM Ellison and NJ Gotelli, Nitrogen availability alters the expression of carnivory in the northern pitcher plant, Sarracenia purpuea. PNAS 99 (2002) 4409-4412
TM Embley and E Stackebrandt, The molecular phylogeny and systematics of the actinomycetes. Annu Rev Microbiol 48 (1994) 257-289
KW Farrish and DF Grigal, Decomposition in an ombrotrophic bog and a minerotrophic fen in Minnesota. Soil Sci 145 (1988) 353-358
MM Fisher, JM Graham and LE Graham, Bacterial abundance and activity across sites within two Northern Wisconsin Sphagnum bogs. Microb Ecol 36 (1998) 259-269
JA Fuerst, The Planctomycetes: emerging models for microbial ecology, evolution and cell biology. Microbiology 141 (1995) 1493-1506
PE Galand, H Fritze and K Yrjälä, Microsite-dependant changes in methanogenic populations in a boreal oligotrophic fen. Environ Microbiol 5 (2003) 1133-1143
S Goodwin and G Zeikus, Ecophysiological adaptations of anaerobic bacteria to low pH: analysis of anaerobic digestion in acidic bog sediments. Appl Environ Microbiol 53 (1987) 57-64
NJ Gotelli and AM Ellison, Biogeography at a regional scale: determinants of ant species density in New England bogs and forests. Ecology 83 (2002) 1604-1609
TC Gsell, WE Holben and RM Ventullo, Characterization of the sediment bacterial community in groundwater discharge zones of an Alkaline Fen: a seasonal study. Appl Environ Microbiol 63 (1997) 3111-3118
Bergey’s Manual of Determinative Bacteriology. Baltimore, MD: Williams and Wilkins (2000).
LC Johnson, WH Damman and N Malmer, Sphagnum macrostructure as an indicator of decay and compaction in peat cores from an ombrotrophic South Swedish peat bog. J Ecol 78 (1990) 633-647
CW Kaplan and CL Kitts, Variation between observed and true terminal restriction fragment length is dependant on true TRF length and purine content. J Microbiol Methods 54 (2003) 121-125
AD Kent, DJ Smith, BJ Benson and EW Triplett, Web-based phylogenetic assignment tool for analysis of terminal restriction fragment length polymorphism profiles of microbial communities. Appl Environ Microbiol 69 (2003) 6768-6776
Y Koizumi, H Kojima, K Oguri, H Kitazato and M Fukui, Vertical and temporal shifts in microbial communities in the water column and sediment of saline meromictic Lake Kaiike (Japan), asdetermined by a 16S rDNA-based analysis, and related to physicochemical gradients. Environ Microbiol 6 (2004) 622-637
CR Kuske, LO Ticknor, ME Miller, JM Dunbar, JA Davis, SM Barns and J Belnap, Comparison of soil bacterial communities in rhizospheres of three plant species and the interspaces in an arid grassland. Appl Environ Microbiol 68 (2002) 1854-1863
MG LaMontagne, I Leifer, S Bergmann, LC Werfhorst Van De and PA Holden, Bacterial diversity in marine hydrocarbon seep sediments. Environ Microbiol 6 (2004) 799
W-T Liu, TL Marsh, H Cheng and LJ Forney, Characterization of microbial diversity by determining terminal restriction fragment length polymorphisms of genes encoding 16S rRNA. Appl Environ Microbiol 63 (1997) 4516-4522
A Loy, K Küsel, A Lehner, HL Drake and M Wagner, Microarray and functional gene analyses of sulfate-reducing prokaryotes in low-sulfate, acidic fens reveal coocurrence of recognized and novel lineages. Appl Environ Microbiol 70 (2004) 6998-7009
MT Madigan, JM Martinko and J Parker, Brock Biology of Microorganisms. Englewood, NJ: Prentice-Hall (2003).
WJ Mitsch and JG Gosselink, Wetlands. New York: Wiley (2000).
Mouser, PJ, Hession, WC, Rizzo, DM, Gotelli, NJ (2005) Hydrology and geostatistics of a Vermont, USA kettlehole peatland. J Hydrol 301: 250–266
S Rendulic, P Jagtap, A Rosinus, M Eppinger, C Baar, C Lanz, H Keller, C Lambert, J Evans, A Goesmann, F Meyer, RE Sockett and SC Schuster, A predator unmasked: life cycle of Bdellovibrio bacteriovorus from a genomic perspective. Science 303 (2004) 689-692
M Sait, P Hugenholtz and PH Janssen, Cultivation of globally distributed soil bacteria from phylogenetic lineages previously only detected in cultivation-independent surveys. Environ Microbiol 4 (2002) 654
Sciarini, SM, Michel, F (2002) tRFLP Fragment Sorter. [2.5b3]. Computer Program
MV Sizova, NS Panikov, TP Tourova and PW Flanagan, Isolation and characterization of oligotrophic acido-tolerant methanogenic consortia from a Sphagnum peat bog. FEMS Microbiol Ecol 45 (2003) 301-315
M Strous, JG Kuenen, JA Fuerst, M Wagner and MS Jetten, The anammox case—a new experimental manifesto for microbiological eco-physiology. Antonie Van Leeuwenhoek 81 (2002) 693-702
M Upton, B Hill, C Edwards, JR Saunders, DA Ritchie and D Lloyd, Combined molecular ecological and confocal laser scanning microscopic analysis of peat bog methanogen populations. FEMS Microbiol Lett 193 (2000) 275-281
LA Niftrik van, JA Fuerst, JSS Damsté, JG Kuenen, MSM Jetten and M Strous, The anammoxosome: an intracytoplasmic compartment in anammox bacteria. FEMS Microbiol Lett 233 (2004) 7-13
JTA Verhoeven, E Maltby and MB Schmitz, Nitrogen and phosphorous mineralization in fens and bogs. J Ecol 78 (1990) 713-726
RT Williams and RL Crawford, Effects of various physiochemical factors on microbial activity in peatlands: aerobic biodegradative processes. Can J Microbiol 29 (1983) 1430-1437
RT Williams and RL Crawford, Microbial diversity of Minnesota peatlands. Microb Ecol 9 (1983) 201-214
H Yamamoto, A Hiraishi, K Kato, HX Chiura, Y Maki and A Shimizu, Phylogenetic evidence for the existence of novel thermophilic bacteria in hot sulfur-turf microbial mats in Japan. Appl Environ Microbiol 64 (1998) 1680-1687
Acknowledgments
This work was supported by funding from NSF (EPS-0082977). The authors would like to thank Aaron M. Ellison for sharing data as well as his extensive knowledge of bog ecology and geography. We are grateful to Don Stratton for assistance with the statistical analysis and Carol Tudhope and Grant Henderson for sample preparation and data organization. Kevin Penn is thanked for performing DNA sequencing and analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morales, S.E., Mouser, P.J., Ward, N. et al. Comparison of Bacterial Communities in New England Sphagnum Bogs Using Terminal Restriction Fragment Length Polymorphism (T-RFLP). Microb Ecol 52, 34–44 (2006). https://doi.org/10.1007/s00248-005-0264-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-005-0264-2