Abstract
Background
Concern regarding gadolinium deposition in the brain after repeated administration of intravenous gadolinium-based contrast agents has prompted evaluation of imaging alternatives.
Objective
The study purpose was to determine if magnetic resonance imaging (MRI) using conventional sequences with diffusion-weighted imaging (DWI) instead of gadolinium-based contrast-enhanced MRI is valid for local staging and guiding biopsies in osseous sarcomas.
Materials and methods
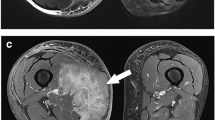
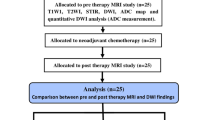
Initial pretreatment MRI with DWI and gadolinium-based contrast-enhanced images in patients ≤ 18 years with histopathologically proven osseous sarcomas were included. Two radiologists blinded to collated demographic and clinical data, independently reviewed conventional/DWI and conventional/gadolinium-based contrast-enhanced MRI then conventional sequences alone, recording tumor size, skip lesions, necrosis, neurovascular invasion, enlarged lymph nodes and diffusion restriction. Discrepancies were resolved by a third reader. A single reader measured apparent diffusion coefficient (ADC) values in non-necrotic tumors, then correlated minimum ADC values -- with and without normalization to skeletal muscle -- with relative enhancement.
Results
Twenty-one patients (mean age: 11.3±4.2 years, 15 [71%] females) had 14 osteosarcomas and 7 Ewing sarcomas, 50% centered in the femur. Conventional/DWI versus conventional/gadolinium-based contrast-enhanced MRI showed agreement for tumor size estimation with significant associations for necrosis (P=0.021), neurovascular involvement (P<0.001) and enlarged lymph nodes (P=0.005). Diagnostic accuracy of conventional/DWI is comparable to conventional/gadolinium-based contrast-enhanced MRI and superior to conventional sequences alone. Comparison between minimum ADC values and relative enhancement showed no correlation (P>0.05).
Conclusion
Significant associations of key imaging features in the initial assessment of osseous sarcomas support DWI as an alternative to gadolinium-based contrast-enhanced MRI. The lack of association between ADC values and relative enhancement suggests that they measure independent constructs, DWI dependent upon tumor cellularity and perfusion.



Similar content being viewed by others
References
Anderson ME (2016) Update on survival in osteosarcoma. Orthop Clin North Am 47:283–292
Ottaviani G, Jaffe N (2009) The epidemiology of osteosarcoma. In: Pediatric and adolescent osteosarcoma. Springer, Boston, pp 3–13
Ward E, DeSantis C, Robbins A et al (2014) Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin 64:83–103
McDonald RJ, McDonald JS, Kallmes DF et al (2015) Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 275:772–782
Hitomi E, Simpkins AN, Luby M et al (2018) Blood–ocular barrier disruption in acute stroke patients. Neurology 90:e915–e923
Maeda H, Wu J, Sawa T et al (2000) Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release 65:271–284
Chavhan GB, AlSabban Z, Babyn PS (2014) Diffusion-weighted imaging in pediatric body MR imaging: principles, technique, and emerging applications. Radiographics 34:E73–E88
Gerrand CH, Rankin K (2014) A system for the surgical staging of musculoskeletal sarcoma. In: Classic papers in orthopaedics. Springer, London, pp 487–488
Subhawong TK, Jacobs MA, Fayad LM (2014) Diffusion-weighted MR imaging for characterizing musculoskeletal lesions. Radiographics 34:1163–1177
Caro-Domínguez P, Gupta AA, Chavhan GB (2018) Can diffusion-weighted imaging distinguish between benign and malignant pediatric liver tumors? Pediatr Radiol 48:85–93
Ma GM, Amirabadi A, Inarejos E et al (2015) MRI thresholds for discrimination between normal and mild temporomandibular joint involvement in juvenile idiopathic arthritis. Pediatr Rheumatol Online 13:53
Taylor R (1990) Interpretation of the correlation coefficient: a basic review. J Diagn Med Sonogr 6:35–39
Bajpai J, Gamnagatti S, Kumar R et al (2011) Role of MRI in osteosarcoma for evaluation and prediction of chemotherapy response: correlation with histological necrosis. Pediatr Radiol 41:441–450
Oka K, Yakushiji T, Sato H et al (2010) The value of diffusion-weighted imaging for monitoring the chemotherapeutic response of osteosarcoma: a comparison between average apparent diffusion coefficient and minimum apparent diffusion coefficient. Skeletal Radiol 39:141–146
Hayashida Y, Yakushiji T, Awai K et al (2006) Monitoring therapeutic responses of primary bone tumors by diffusion-weighted image: initial results. Eur Radiol 16:2637–2643
Kitajima K, Kaji Y, Fukabori Y et al (2010) Prostate cancer detection with 3 T MRI: comparison of diffusion weighted imaging and dynamic contrast-enhanced MRI in combination with T2-weighted imaging. J Magn Reson Imaging 31:625–631
Kuang F, Yan Z, Li H, Feng H (2015) Diagnostic accuracy of diffusion weighted MRI for differentiation of cervical cancer and benign cervical lesions at 3.0 T: comparison with routine MRI and dynamic contrast enhanced MRI. J Magn Reson Imaging 42:1094–1099
Kitajima K, Tanaka U, Ueno Y et al (2015) Role of diffusion weighted imaging and contrast-enhanced MRI in the evaluation of intrapelvic recurrence of gynecological malignant tumor. PLoS One 10:e0117411
Vijayakumar V, Collier AB 3rd, Ruan C et al (2016) Multimodality imaging in pediatric osteosarcoma in the era of image gently and image wisely campaign with a close look at the CT scan radiation dose. J Pediatr Hematol Oncol 38:227–231
Le Bihan D (2008) Intravoxel incoherent motion perfusion MR imaging: a wake-up call. Radiology 249:748–752
Freiman M, Perez-Rossello JM, Callahan MJ et al (2013) Characterization of fast and slow diffusion from diffusion weighted MRI of pediatric Crohn's disease. J Magn Reson Imaging 37:156–163
Giles SL, Morgan VA, Riches SF et al (2011) Apparent diffusion coefficient as a predictive biomarker of prostate cancer progression: value of fast and slow diffusion components. AJR Am J Roentgenol 196:586–591
Shinmoto H, Oshio K, Tanimoto A et al (2009) Biexponential apparent diffusion coefficients in prostate cancer. Magn Reson Imaging 27:355–359
deSouza NM, Riches SF, Vanas NJ et al (2008) Diffusion-weighted magnetic resonance imaging: a potential non-invasive marker of tumour aggressiveness in localized prostate cancer. Clin Radiol 63:774–782
Song JS, Hwang SB, Chung GH, Jin GY (2016) Intra-individual, inter-vendor comparison of diffusion weighted MR imaging of upper abdominal organs at 3.0 Tesla with an emphasis on the value of normalization with the spleen. Korean J Radiol 17:209–217
Papanikolaou N, Gourtsoyianni S, Yarmenitis S et al (2010) Comparison between two-point and four-point methods for quantification of apparent diffusion coefficient of normal liver parenchyma and focal lesions. Value of normalization with spleen. Eur J Radiol 73:305–309
Acknowledgements
This manuscript was presented as a scientific paper at the 2018 Society for Pediatric Radiology (SPR) Meeting in Nashville, TN. The authors would like to acknowledge Mr. Warren Corber, research assistant, for his contribution in the randomization and anonymization of our patient cohort and data recording.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alsharief, A.N., Martinez-Rios, C., Hopyan, S. et al. Usefulness of diffusion-weighted MRI in the initial assessment of osseous sarcomas in children and adolescents. Pediatr Radiol 49, 1201–1208 (2019). https://doi.org/10.1007/s00247-019-04436-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-019-04436-y