Abstract
Purpose
The one-dose daily regime of rivaroxaban could cause a pronounced variability in concentration and effect of which a deeper knowledge is warranted. This study aimed to evaluate the typical exposure range and effect of the direct factor Xa (FXa)-inhibitor rivaroxaban in a cohort of well-characterized patients with atrial fibrillation (AF).
Methods
Seventy-one AF patients (72 ± 8 years, 55 % men) were treated with rivaroxaban 15 mg/20 mg (n = 10/61) OD. Trough (n = 71) and peak (n = 30) plasma concentrations determined by liquid chromatography-tandem mass-spectrometry (LC-MS/MS) were compared to the coagulation assays anti-FXa for rivaroxaban, prothrombin time-international normalized ratio (PT-INR) (venous samples and point-of-care assay (POC) CoaguChek XS Pro), and aPTT.
Results
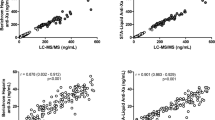
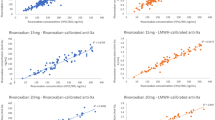
Median rivaroxaban plasma concentrations by LC-MS/MS were 34 (range 5–84) and 233 ng/ml (range 120–375) at trough and peak, respectively. A strong correlation between LC-MS/MS and the anti-FXa assay was found (p < 0.001) for both trough (r 2 = 0.92) and peak (r 2 = 0.91) samples. PT-INR results from the POC assay, but not from the conventional PT assay, correlated significantly with LC-MS/MS in peak samples exclusively (r 2 = 0.41, p < 0.001).
Conclusions
In “real-life” AF patients treated with rivaroxaban, we observed a pronounced variability in plasma concentrations at trough and to a lesser extent at peak measured by LC-MS/MS. The anti-FXa assay performed well upon rivaroxaban levels in a normal exposure range, although LC-MS/MS remains the only method that covers the whole concentration range with accuracy. Interestingly, the POC assay for PT-INR could be useful to indicate high exposure to rivaroxaban in emergency situations although further validation is required.



Similar content being viewed by others
References
Patel MR, Mahaffey KW, Garg J, et al. (2011) Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 365(10):883–891. doi:10.1056/NEJMoa1009638
Antovic JP, Skeppholm M, Eintrei J, et al. (2013) Evaluation of coagulation assays versus LC-MS/MS for determinations of dabigatran concentrations in plasma. Eur J Clin Pharmacol 69(11):1875–1881. doi:10.1007/s00228-013-1550-4
Skeppholm M, Al-Aieshy F, Berndtsson M, et al. (2015) Clinical evaluation of laboratory methods to monitor apixaban treatment in patients with atrial fibrillation. Thromb Res 136(1):148–153. doi:10.1016/j.thromres.2015.04.030
Reilly PA, Lehr T, Haertter S, et al. (2014) The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: the RE-LY Trial (Randomized Evaluation of Long-Term Anticoagulation Therapy). J Am Coll Cardiol 63(4):321–328. doi:10.1016/j.jacc.2013.07.104
Camm AJ, Lip GY, De Caterina R, et al. (2012) 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 33(21):2719–2747. doi:10.1093/eurheartj/ehs253
Heidbuchel H, Verhamme P, Alings M, et al. (2015) Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace. doi:10.1093/europace/euv309
European Medicines Agency. Summary of product characteristics, Xarelto (rivaroxaban). Bayer Pharma AG. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000944/WC500057108.pdf. Accessed september 28 2015
Cuker A, Siegal DM, Crowther MA, et al. (2014) Laboratory measurement of the anticoagulant activity of the non-vitamin K oral anticoagulants. J Am Coll Cardiol 64(11):1128–1139. doi:10.1016/j.jacc.2014.05.065
Samama MM, Contant G, Spiro TE, et al. (2013) Laboratory assessment of rivaroxaban: a review. Thromb J 11(1):11. doi:10.1186/1477-9560-11-11
Perzborn E, Strassburger J, Wilmen A, et al. (2005) In vitro and in vivo studies of the novel antithrombotic agent BAY 59-7939–an oral, direct Factor Xa inhibitor. J Thromb Haemost 3(3):514–521. doi:10.1111/j.1538-7836.2005.01166.x
Freyburger G, Macouillard G, Khennoufa K, et al. (2015) Rivaroxaban and apixaban in orthopaedics: is there a difference in their plasma concentrations and anticoagulant effects? Blood Coagul Fibrinolysis. doi:10.1097/MBC.0000000000000371
Douxfils J, Tamigniau A, Chatelain B, et al. (2013) Comparison of calibrated chromogenic anti-Xa assay and PT tests with LC-MS/MS for the therapeutic monitoring of patients treated with rivaroxaban. Thromb Haemost 110(4):723–731. doi:10.1160/TH13-04-0274
Samama MM, Martinoli JL, LeFlem L, et al. (2010) Assessment of laboratory assays to measure rivaroxaban–an oral, direct factor Xa inhibitor. Thromb Haemost 103(4):815–825. doi:10.1160/TH09-03-0176
European Medicines Agency. Guideline on bioanalytical method validation EMEA/CHMP/EWP/192217/2009, Committee for Medicinal Products for Human Use (CHMP). http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/08/WC500109686.pdf. Accessed October 5, 2015
Francart SJ, Hawes EM, Deal AM, et al. (2014) Performance of coagulation tests in patients on therapeutic doses of rivaroxaban. A cross-sectional pharmacodynamic study based on peak and trough plasma levels. Thromb Haemost 111(6):1133–1140. doi:10.1160/TH13-10-0871
Ebner M, Peter A, Spencer C, et al. (2015) Point-of-care testing of coagulation in patients treated with non-vitamin K antagonist oral anticoagulants. Stroke 46(10):2741–2747. doi:10.1161/STROKEAHA.115.010148
Mueck W, Borris LC, Dahl OE, et al. (2008) Population pharmacokinetics and pharmacodynamics of once- and twice-daily rivaroxaban for the prevention of venous thromboembolism in patients undergoing total hip replacement. Thromb Haemost 100(3):453–461
Mueck W, Lensing AW, Agnelli G, et al. (2011) Rivaroxaban: population pharmacokinetic analyses in patients treated for acute deep-vein thrombosis and exposure simulations in patients with atrial fibrillation treated for stroke prevention. Clin Pharmacokinet 50(10):675–686. doi:10.2165/11595320-000000000-00000
FDA. Center for drug evaluation and research, application number 022406Orig1s000. Clinical pharmacology and biopharmaceutics review (s) http://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/022406Orig1s000ClinPharmR.pdf. Accessed October 5 2015
Bardy G, Fischer F, Appert A, et al. (2015) Is anti-factor Xa chromogenic assay for Rivaroxaban appropriate in clinical practice? Advantages and comparative drawbacks. Thromb Res 136(2):396–401. doi:10.1016/j.thromres.2015.05.015
Konigsbrugge O, Quehenberger P, Belik S, et al. (2015) Anti-coagulation assessment with prothrombin time and anti-Xa assays in real-world patients on treatment with rivaroxaban. Ann Hematol 94(9):1463–1471. doi:10.1007/s00277-015-2407-y
Mani H, Herth N, Kasper A, et al. (2014) Point-of-care coagulation testing for assessment of the pharmacodynamic anticoagulant effect of direct oral anticoagulant. Ther Drug Monit 36(5):624–631. doi:10.1097/FTD.0000000000000064
Hillarp A, Baghaei F, Fagerberg Blixter I, et al. (2011) Effects of the oral, direct factor Xa inhibitor rivaroxaban on commonly used coagulation assays. J Thromb Haemost 9(1):133–139. doi:10.1111/j.1538-7836.2010.04098.x
Heidbuchel H, Verhamme P, Alings M, et al. (2013) European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace 15(5):625–651. doi:10.1093/europace/eut083
Summary of product characteristics, Lasix Retard (furosemide). Sanofi. http://www.fass.se/LIF/product?35&userType=0&nplId=19820429000034&docType=6. Accessed November 1 2015
Summary of product characteristics, Aldactone (spironolactone). Pfizer. http://www.fass.se/LIF/product?42&userType=0&nplId=19800613000071&docType=6. Accessed November 1 2015
FDA. Prescribing information. Reference ID: 3039818. http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202439s000lbl.pdf. Accessed October 22 2015
Acknowledgments
The authors are grateful toward Annika Östlund and Lisbeth Söderblom for their help with the laboratory methods, and toward Lena Gabrielsson for skilful care of the study population. This study was supported by grants from The Coagulation Research fund of Karolinska Institutet, the Foundation Sigurd, and Elsa Goljes memory; from the Stockholm County Council and its Drug and Therapeutics Committee; from the Swedish Heart-Lung Foundation; and from The Swedish Society of Medicine.
Contributions to the manuscript
FAA—patient inclusion, data analyses, literature search, and writing and reviewing the manuscript
REM—responsible for the study design. Performed data analyses and interpretation and writing and reviewing the manuscript
JA—responsible for setup and validation of the rivaroxaban anti-Xa assay and reviewing the manuscript
AP—responsible for setup and validation of LC-MS/MS assay for rivaroxaban and writing the corresponding methods section of the manuscript
YR—setup and validation of LC-MS/MS assay for rivaroxaban and writing the corresponding methods section of the manuscript
MB—setup and validation of the rivaroxaban anti-Xa assay and reviewing the manuscript
FAK—recruiting patients and reviewing the manuscript
MS—responsible for the study design, patient inclusion, and sampling. Performed data analysis and interpretation and writing and reviewing the manuscript
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was performed in accordance with the Declaration of Helsinki and was approved by the Ethical Review Board in Stockholm, Sweden. The subjects were enrolled from the coagulation centre at Danderyd’s Hospital in the Stockholm County, during December 4, 2012, to December 19, 2014, and before participating, oral and written informed consent was attained
ᅟ
Conflicts of interest
Dr. F Al-Khalili received honorarium for acting as speaker for Bayer, Boehringer Ingelheim, and Pfizer. Dr. J Antovic has received speakers’ honoraria and support for attendance at scientific meetings from Stago. None of the other authors declare any conflict of interest related to this work.
Electronic Supplementary Material
Supplementary figure 1
Correlation between individual trough and peak rivaroxaban plasma concentrations (15 and 20 mg OD, n = 30) measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS). (PPTX 82 kb)
Supplementary figure 2
Correlation between rivaroxaban trough plasma concentrations measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS) and glomerular filtration rate estimated by the Cockcroft-Gault formula (CCG) (20 mg OD, n = 61). The regression line is added. (PPTX 70 kb)
Supplementary figure 3
Rivaroxaban trough plasma concentrations (20 mg OD, n = 61) measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS) in relation to CHADS2 score (0–1 and ≥2). The box plots show median, upper and lower quartile with extension to points still within 1.5 interquartile range from the quartiles, points farther away are shown as outliers. (PPTX 66 kb)
Rights and permissions
About this article
Cite this article
Al-Aieshy, F., Malmström, R.E., Antovic, J. et al. Clinical evaluation of laboratory methods to monitor exposure of rivaroxaban at trough and peak in patients with atrial fibrillation. Eur J Clin Pharmacol 72, 671–679 (2016). https://doi.org/10.1007/s00228-016-2060-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-016-2060-y