Abstract
Objectives
To explore the effect of cytochrome P450 2C19 (CYP2C19) polymorphisms on the relationship between the pharmacokinetics and pharmacodynamics of omeprazole administered by intravenous successive infusions in Chinese healthy volunteers.
Methods
A total of 21 subjects [7 homozygous extensive metabolizers (homEMs), 9 heterozygous extensive metabolizers (hetEMs), 5 poor metabolizers (PMs)] received a 5-day course of omeprazole (40 mg) administered as a single dose daily during a 30-min period. Plasma concentrations were monitored by sampling at very short intervals for the first 8.5 h post-omeprazole administration and at 24 h post-administration, and intragastric pH was recorded on days 1 and 5.
Results
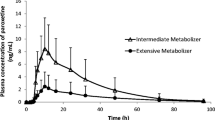
After a single dose, both the area under the plasma concentration–time curve (AUC) and peak concentration (Cmax) were higher in PMs than in EMs. Both the mean half-life (t½) and total clearance in PMs were significantly higher and lower than those in homEMs and EMs, respectively. Mean AUC and Cmax ratios in homEMs, hetEMs, and PMs were 1.0:1.1:1.4 and 1.0:1.0:1.1, respectively. Relative to the values determined after a single dose in EMs, after repeated doses, the intragastric pH, AUC, Cmax, and t½ had increased significantly, while the total clearance had decreased significantly. Mean AUC and Cmax ratios in homEMs, hetEMs, and PMs were 1.4:1.4:1.5 and 1.2:1.2:1.3, respectively, compared to those of a single dose. The mean intragastric pH was significantly higher in PMs than in EMs after the fifth dose.
Conclusions
There is a relationship between the pharmacokinetics and pharmacodynamics of omeprazole, with the latter depending in part on the duration of administration as evidenced by a higher AUC or Cmax and intragastric pH resulting from repeated dosing.



Similar content being viewed by others
Reference
Desta Z, Zhao X, Shin JG, Flockhart DA (2002) Clinical significance of the cytochrome P450 2C19 genetic polymorphism. Clin Pharmacokinet 41:913–958
Sagar M, Tybring G, Dahl ML, Bertilsson L, Seensalu R (2000) Effects of omeprazole on intragastric pH and plasma gastrin are dependent on the CYP2C19 polymorphism. Gastroenterology 119:670–676
Tsukasa U, Takenori N, Makoto H, Norio YF, Kazunobu S, Tomonori T (2007) Absolute bioavailability and metabolism of omeprazole in relation to CYP2C19 genotypes following single intravenous and oral administrations. Eur J Clin Pharmacol 63:143–149
Ando T, Ishikawa T, Kokura S, Naito Y, Yoshida N, Yoshikawa T (2008) Endoscopic analysis of gastric ulcer after one week’s treatment with omeprazole and rabeprazole in relation to CYP2C19 genotype. Dig Dis Sci 53:933–937
Klotz U, Schwab M, Treiber G (2004) CYP2C19 polymorphism and proton pump inhibitors. Basic Clin Pharmacol Toxicol 95:2–8
Niioka T, Uno T, Sugimoto K, Sugawara K, Hayakari M, Tateishi T (2007) Estimation of CYP2C19 activity by the omeprazole hydroxylation index at a single point in time after intravenous and oral administration. Eur J Clin Pharmacol 63:1031–1038
Klotz U (2006) Clinical impact of CYP2C19 polymorphism on the action of proton pump inhibitors: a review of a special problem. Int J Clin Pharmacol Ther 44:297–302
Hu XP, Xu JM, Hu YM, Mei Q, Xu XH (2007) Effects of CYP2C19 genetic polymorphism on the pharmacokinetics and pharmacodynamics of omeprazole in Chinese people. J Clin Pharmacol Ther 32(5):517–524
Kurzawski M, Gawronska-Szklarz B, Wrzesniewska J, Siuda A, Starzynska T, Drozdzik M (2006) Effect of CYP2C19*17 gene variant on Helicobacter pylori eradication in peptic ulcer patients. Eur J Clin Pharmacol 62:877–880
Padol S, Yuan Y, Thabane M, Padol IT, Hunt RH (2006) The effect of CYP2C19 polymorphisms on H. pylori eradication rate in dual and triple first-line PPI therapies: a meta-analysis. Am J Gastroenterol 101:1467–1475
Shirai N, Furuta T, Moriyama Y, Okochi H, Kobayashi K, Takashima M, Xiao F, Kosuge K, Nakagawa K (2001) Effects of CYP2C19 genotypic differences in the metabolism of omeprazole and rabeprazole on intragastric pH. Aliment Pharmacol Ther 15(12):1929–1937
Rudberg I, Mohebi B, Hermann M, Refsum H, Molden E (2008) Impact of the ultrarapid CYP2C19*17 allele on serum concentration of escitalopram in psychiatric patients. Mol Ther 83:322–327
Norio YF, Takenori T, Taku N, Gen Y, Yoshimasa I, Sunao K, Tomonori T (2004) Different inhibitory effect of fluvoxamine on omeprazole metabolism between CYP2C19 genotypes. Br J Clin Pharmacol 57(4):487–494
DSc GS, Shin JM, Vagin O, Lambrecht N, Yakubov I, Munson K (2007) The gastric H, K ATPase as a drug target: past, present, and future. J Clin Gastroenterol 41:S226–S242
Baldwin RM, Ohlsson S, Pedersen RS, Mwinyi J, Ingelman-Sundberg M, Eliasson E, Bertilsson L (2008) Increased omeprazole metabolism in carriers of the CYP2C19*17 allele; a pharmacokinetic study in healthy volunteers. Br J Clin Pharmacol 65(5):767–774
Sim SC, Risinger C, Dahl ML, Aklillu E, Christensen M, Bertilsson L, Ingelman-Sundberg M (2006) A common novel CYP2C19 gene variant causes ultrarapid drug metabolism relevant for the drug response to proton pump inhibitors and antidepressants. Clin Pharmacol Ther 79:103–113
Horn J (2004) Relationship between the metabolism and efficacy of proton pump inhibitors – focus on rabeprazole. Aliment Pharmacol Ther 20[Suppl 6]:11–19
Qiao HL, Hu YR, Tian X, Jia LJ, Gao N, Zhang LR, Guo YZ (2006) Pharmacokinetics of three proton pump inhibitors in Chinese subjects in relation to the CYP2C19 genotype. Eur J Clin Pharmacol 62:1–6
Kirchheiner J, Nickchen K, Bauer M, Wong ML, Licinio J, Roots I, Brockmoller J (2004) Pharmacogenetics of antidepressants and antipsychotics: the contribution of allelic variations to the phenotype of drug response. Mol Psychiatr 9:442–473
Takada K, Arefayene M, Desta Z, Yarboro CH, Boumpas DT, Balow JE, Flockhart DA, Illei GG (2004) Cytochrome P450 pharmacogenetics as a predictor of toxicity and clinical response to pulse cyclophosphamide in lupus nephritis. Arthritis Rheum 50:2202–2210
Li Y, Hou J, Jiang H, Wang D, Fu W, Yuan Z, Chen Y, Zhou L (2007) Polymorphisms of CYP2C19 gene are associated with the efficacy of thalidomide based regimens in multiple myeloma. Haematol 92:1246–1249
Bottiger Y (2006) Use of omeprazole sulfone in a single plasma sample as a probe for CYP3A4. Eur J Clin Pharmacol 62:621–625
Walton R, Kimber M, Rockett K, Trafford C, Kwiatkowski D, Sirugo G (2005) Haplotype block structure of the cytochrome P450 CYP2C gene cluster on chromosome 10. Nat Genet 37:915–916
Acknowledgments
We thank Mr. Yaozong Yuan, Ruijin Hospital affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, Mr. Jianming Xu, First Affiliated Hospital of Anhui Medical University, Anhui, and Mrs. Hongwei Fan, Nanjing First Hospital affiliated to Nanjing Medical University, Nanjing for providing some of the plasma and intragastric pH value samples. This study was supported by Nanjing Changao Pharmaceutical Science & Technology Co., Nanjing, China.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, Y., Zhang, H., Meng, L. et al. Influence of CYP2C19 on the relationship between pharmacokinetics and intragastric pH of omeprazole administered by successive intravenous infusions in Chinese healthy volunteers. Eur J Clin Pharmacol 66, 563–569 (2010). https://doi.org/10.1007/s00228-010-0821-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-010-0821-6