Abstract
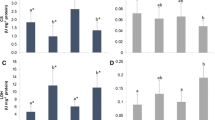
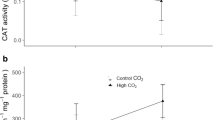
Ocean acidification is a consequence of chemical changes driven mainly by a continuous uptake of carbon dioxide, resulting in pH decrease. This phenomenon represents an additional threat to marine life, with expected effects ranging from changes in behavioral responses and calcification rates to the potential promotion of oxidative stress. To unravel the impacts of ocean acidification on the antioxidant system of sharks, we performed a long-term exposure (9 months, since early embryogenesis) to high CO2 conditions (pCO2 ~ 900 μatm) on a temperate shark (Scyliorhinus canicula). The following biomarkers were measured: enzymatic antioxidant defense (superoxide dismutase, catalase and glutathione peroxidase), protein repair and removal (heat shock proteins and ubiquitin), and oxidative damage on lipids (malondialdehyde) and DNA (8-hydroxy-2′-deoxyguanosine). Changes in the antioxidant enzyme defense were restricted to an increase in catalase activity in the muscle, an enzyme that plays a major role in oxidative stress mitigation. On the other hand, no evidence of oxidative damage was found, indicating that the observed increase in catalase activity may be enough to neutralize the effects of potentially higher reactive oxygen species. These results further indicate that these sharks’ antioxidant system can successfully cope with the levels of carbon dioxide projected for the end of the century. Nonetheless, the interaction between ocean acidification and the rise in temperature expected to occur in a near future may disturb their antioxidant capacity, requiring further investigation.



Similar content being viewed by others
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Andreyev AY, Kushnareva YE, Starkov AA (2005) Mitochondrial metabolism of reactive oxygen species. Biochemistry 70:200–214
Baldwin J, Wells RMG (1990) Oxygen transport potential in tropical elasmobranchs from the Great Barrier Reef: relationship between haematology and blood viscosity. J Exp Mar Biol Ecol 144:145–155
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1-2):248–254
Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O (2012) Oxidative stress and antioxidant defense. World Allergy Organ J 5:9–19
Bockus AB, Seibel BA (2018) Synthetic capacity does not predict elasmobranchs' ability to maintain trimethylamine oxide without a dietary contribution. Comp Biochem Physiol A Mol Integr Physiol 217:35–42
Bond U, Agell N, Haas AL, Redman K, Schlesinger MJ (1988) Ubiquitin in stressed chicken embryo fibroblasts. J Biol Chem 263:2384–2388
Cadenas E (1989) Biochemistry of oxygen toxicity. Annu Rev Biochem 58:79–110
Caldeira K, Wickett ME (2003) Oceanography: anthropogenic carbon and ocean pH. Nature 425:365
Dickson AG, Millero FJ (1987) A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Res Part A Oceanogr Res Pap 34:1733–1743
Dixson DL, Jennings AR, Atema J, Munday PL (2015) Odor tracking in sharks is reduced under future ocean acidification conditions. Glob Chang Biol 21:1454–1462
Doney SC, Fabry VJ, Feely RA, Kleypas JA (2009) Ocean acidification: the other CO2 problem. Annu Rev Mar Sci 1:169–192
Doney SC, Ruckelshaus M, Duffy JE, Barry JP, Chan F, English CA, Galindo HM, Grebmeier JM, Hollowed AB, Knowlton N (2012) Climate change impacts on marine ecosystems. Annu Rev Mar Sci 4(1):11–37
Dupont S, Dorey N, Stumpp M, Melzner F, Thorndyke M (2013) Long-term and trans-life-cycle effects of exposure to ocean acidification in the green sea urchin Strongylocentrotus droebachiensis. Mar Biol 160:1835–1843
Felzenszwalb I, de Mattos JCP, Bernardo-Filho M, Caldeira-de-Araújo A (1998) Shark cartilage-containing preparation: protection against reactive oxygen species. Food Chem Toxicol 36:1079–1084
Fridovich I (1983) Superoxide radical: an endogenous toxicant. Annu Rev Pharmacol Toxicol 23:239–257
Gattuso J-P, Hansson L (2011) Ocean acidification: background and history. Oxford University Press, Oxford, pp 1–20
Gibson R, Atkinson R, Gordon J, Smith I, Hughes D (2011) Impact of ocean warming and ocean acidification on marine invertebrate life history stages: vulnerabilities and potential for persistence in a changing ocean. Ocean Mar Biol Annu Rev 49:1–42
Gomes EM, Souto PRF, Felzenszwalb I (1996) Shark-cartilage containing preparation protects cells against hydrogen peroxide induced damage and mutagenesis. Mutat Res Toxicol 367:203–208
Green L, Jutfelt F (2014) Elevated carbon dioxide alters the plasma composition and behaviour of a shark. Biol Lett 10:20140538–20140538
Halliwell B, Gutteridge JMC (2015) Free radicals in biology and medicine. Oxford University Press, Oxford
Hanna J, Meides A, Zhang DP, Finley D (2007) A ubiquitin stress response induces altered proteasome composition. Cell 129:747–759
Hanschmann EM, Godoy JR, Berndt C, Hudemann C, Lillig CH (2013) Thioredoxins, glutaredoxins, and peroxiredoxins—molecular mechanisms and health significance: from cofactors to antioxidants to redox signaling. Antioxid Redox Signal 19:1539–1605
Haugan PM, Drange H (1996) Effects of CO2 on the ocean environment. Energy Convers Manage 37(6–8):1019–1022
Heinrich DDU, Rummer JL, Morash AJ, Watson SA, Simpfendorfer CA, Heupel MR, Munday PL (2014) A product of its environment: the epaulette shark (Hemiscyllium ocellatum) exhibits physiological tolerance to elevated environmental CO2. Conserv Physiol 2:1–12
Hendriks IE, Duarte CM, Álvarez M (2010) Vulnerability of marine biodiversity to ocean acidification: a meta-analysis. Estuar Coast Shelf Sci 86:157–164
Heuer RM, Grosell M (2014) Physiological impacts of elevated carbon dioxide and ocean acidification on fish. Am J Physiol Regul Integr Comp Physiol 307(9):R1061–R1084
Hochachka PW, Somero GN (2002) Biochemical adaptation. Oxford University Press, New York
Ighodaro OM, Akinloye OA (2018) First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J Med 54:287–293
IPCC (2014) Climate change 2014: synthesis report. In: Pachauri RK, Meyer LA (eds) Core Writing Team. IPCC, Geneva
Johansson LH, Borg LH (1988) A spectrophotometric method for determination of catalase activity in small tissue samples. Anal Biochem 174:331–336
Johnson MS, Kraver DW, Renshaw GMC, Rummer JL (2016) Will ocean acidification affect the early ontogeny of a tropical oviparous elasmobranch (Hemiscyllium ocellatum)? Conserv Physiol4(1):cow003
Kroeker KJ, Kordas RL, Crim RN, Singh GG (2010) Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol Lett 13:1419–1434
Lawrence RA, Burk RF (1976) Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun 71:952–958
Lesser MP (2006) Oxidative stress in marine environments: biochemistry and physiological ecology. Annu Rev Physiol 68:253–278
Lesser MP (2012) Oxidative stress in tropical marine ecosystems. Oxidative Stress Aquat Ecosyst 1:9–19
Lopes AR, Sampaio E, Santos C, Couto A, Pegado MR, Diniz M, Munday PL, Rummer JL, Rosa R (2018) Absence of cellular damage in tropical newly hatched sharks (Chiloscyllium plagiosum) under ocean acidification conditions. Cell Stress Chaperones 23(5):837–846
López-Cruz RI, Zenteno-Savín T, Galván-Magaña F (2010) Superoxide production, oxidative damage and enzymatic antioxidant defenses in shark skeletal muscle. Comp Biochem Physiol Part A Mol Integr Physiol 156:50–56
Matoo OB, Ivanina AV, Ullstad C, Beniash E, Sokolova IM (2013) Interactive effects of elevated temperature and CO2 levels on metabolism and oxidative stress in two common marine bivalves (Crassostrea virginica and Mercenaria mercenaria). Comp Biochem Physiol Part A Mol Integr Physiol 164:545–553
Matozzo V, Chinellato A, Munari M, Bressan M, Marin MG (2013) Can the combination of decreased pH and increased temperature values induce oxidative stress in the clam Chamelea gallina and the mussel Mytilus galloprovincialis? Mar Pollut Bull 72:34–40
Maulvault AL, Barbosa V, Alves R, Anacleto P, Camacho C, Cunha S, Fernandes JO, Ferreira PP, Rosa R, Marques A (2018) Integrated multi-biomarker responses of juvenile seabass to diclofenac, warming and acidification co-exposure. Aquat Toxicol 202:65–79
McCord JM, Fridovich I (1969) Superoxide dismutase an enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244(22):6049–6055
Mehrbach C, Culberson CH, Hawley JE, Pytkowicx RM (1973) Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol Oceanogr 18:897–907
Njemini R, Lambert M, Demanet C, Mets T (2005) Heat shock protein 32 in human peripheral blood mononuclear cells: effect of aging and inflammation. J Clin Immunol 25:405–417
Orr JC, Fabry VJ, Aumont O, Bopp L, Doney SC, Feely RA, Gnanadesikan A, Gruber N, Ishida A, Joos F et al (2005) Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437(7059):681–686
Pamplona R, Costantini D (2011) Molecular and structural antioxidant defenses against oxidative stress in animals. Am J Physiol Integr Comp Physiol 301:R843–R863
Parsell DA, Lindquist S (1993) The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genet 27:437–496
Pegado MR, Santos CP, Pimentel M, Cyrne R, Paulo M, Maulvaut AL, Raffoul D, Diniz M, Bispo R, Rosa R (2019) Effects of elevated carbon dioxide on the hematological parameters of a temperate catshark. J Exp Zool Part A Ecol Integr Physiol 333:126–132
Pimentel MS, Faleiro F, Diniz M, Machado J, Pousão-Ferreira P, Peck MA, Pörtner HO, Rosa R (2015) Oxidative stress and digestive enzyme activity of flatfish larvae in a changing ocean. PLoS ONE 10:e0134082
Pistevos JCA, Nagelkerken I, Rossi T, Olmos M, Connell SD (2015) Ocean acidification and global warming impair shark hunting behaviour and growth. Sci Rep 5:16293
Ries JB, Cohen AL, McCorkle DC (2009) Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology 37:1131–1134
Rosa R, Baptista M, Lopes VM, Pegado MR, Ricardo Paula J, Trubenbach K, Leal MC, Calado R, Repolho T (2014) Early-life exposure to climate change impairs tropical shark survival. Proc R Soc B Biol Sci 281:20141738–20141738
Rosa R, Pimentel MS, Boavida-Portugal J, Teixeira T, Trübenbach K, Diniz M (2012) Ocean warming enhances malformations, premature hatching, metabolic suppression and oxidative stress in the early life stages of a keystone squid. PLoS ONE 7:e38282
Rosa R, Ricardo Paula J, Sampaio E, Pimentel M, Lopes AR, Baptista M, Guerreiro M, Santos C, Campos D, Almeida-Val VMF et al (2016) Neuro-oxidative damage and aerobic potential loss of sharks under elevated CO2 and warming. Mar Biol 163(5):119
Rosa R, Rummer JL, Munday PL (2017) Biological responses of sharks to ocean acidification. Biol Lett 13:20160796
Samerotte AL, Drazen JC, Brand GL, Seibel BA, Yancey PH (2007) Correlation of trimethylamine oxide and habitat depth within and among species of teleost fish: an analysis of causation. Physiol Biochem Zool 80(2):197–208
Sarazin G, Michard G, Prevot F (1999) A rapid and accurate spectroscopic method for alkalinity measurements in sea water samples. Water Res 33:290–294
Seibel BA, Walsh PJ (2002) Trimethylamine oxide accumulation in marine animals: relationship to acylglycerol storagej. J Exp Biol 205(3):297–306
Sims DW (1994) Physiological factors regulating appetite in the lesser spotted dogfish shark, Scyliorhinus canicula (L.). Dissertation, University of Plymouth, Plymouth, UK
Shen J, Deininger P, Hunt JD, Zhao H (2007) 8-hydroxy-2′- deoxyguanosine (8-OH-dG) as a potential survival biomarker in patients with nonsmall-cell lung cancer. Cancer 109:574–580
Sokolova IM, Sukhotin AA, Lannig G (2012) Stress effects on metabolism and energy budgets in mollusks. In: Abele D, Vazquez-Medina J, Zenteno-Savin T (eds) Oxidative stress in aquatic ecosystems. Wiley-Blackwell. ISBN:978-1-4443-3548-4
Tiedke J, Cubuk C, Burmester T (2013) Environmental acidification triggers oxidative stress and enhances globin expression in zebrafish gills. Biochem Biophys Res Commun 441(3):624–629
Tomanek L (2011) Environmental proteomics: changes in the proteome of marine organisms in response to environmental stress, pollutants, infection, symbiosis, and development. Ann Rev Mar Sci 3:373–399
Tomanek L, Zuzow MJ, Ivanina AV, Beniash E, Sokolova IM (2011) Proteomic response to elevated pCO2 level in eastern oysters, Crassostrea virginica: evidence for oxidative stress. J Exp Biol 214:1836–1844
Uchiyama M, Mihara M (1978) Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86:271–278
Ufnal M, Zadlo A, Ostaszewski R (2015) TMAO: a small molecule of great expectations. Nutrition 31(11–12):1317–1323
Valavanidis A, Vlahogianni T, Dassenakis M, Scoullos M (2006) Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol Environ Saf 64:178–189
Wang Q, Cao R, Ning X, You L, Mu C, Wang C, Wei L, Cong M, Wu H, Zhao J (2016) Effects of ocean acidification on immune responses of the Pacific oyster Crassostrea gigas. Fish Shellfish Immunol 49:24–33
Wang W, Vinocur B, Shoseyov O, Altman A (2004) Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci 9:244–252
Wang X, Wu L, Aouffen M, Mateescu M, Nadeau R, Wang R (1999) Novel cardiac protective effects of urea: from shark to rat. Br J Pharmacol 128:1477–1484
Wittmann AC, Pörtner H-O (2013) Sensitivities of extant animal taxa to ocean acidification. Nat Clim Chang 3:995
Acknowledgements
We would like to thank the Reviewers for improving the manuscript. We also thank Omar Moura and Beatriz Arzeni for helping perform the biochemical analyses.
Funding
This work was supported by Fundação para a Ciência e Tecnologia (FCT), through the strategic project UID/MAR/04292/2013 granted to MARE, a project grant PTDC/AAG-GLO/1926/2014 and Programa Investigador FCT 2013 granted to RR. This work was also supported by the Applied Molecular Biosciences Unit—UCIBIO which is financed by national funds from FCT/MCTES (UID/Multi/04378/2019). This work was further supported through post-doc MP (SFRH/BPD/117533/2016) and PhD grants to MRP (SFRH/BD/111691/2015), CS (SFRH/BD/117890/2016) and ES (SFRH/BD/131771/2017), financed by national and community funds from FCT and the European Social Fund (ESF), through the Human Capital Operating Programme and Regional Operation Programme (Lisboa 2020).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Research involving animals
The authors declare that experimental procedures were in accordance with the European Parliament (Directive 2010/63/EU) and Council of 22 September 2010 requirements regarding animal protection for scientific studies. All procedures were reviewed and approved by the Animal Welfare Body of FCUL (Statement 5/2016), the animal ethics committee ORBEA, and the National Veterinary Medicines Directorate (DGAV).
Additional information
Responsible Editor: H.-O. Pörtner.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewed by D. Moreira and an undisclosed expert.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pegado, M.R., Santos, C.P., Pimentel, M. et al. Lack of oxidative damage on temperate juvenile catsharks after a long-term ocean acidification exposure. Mar Biol 167, 165 (2020). https://doi.org/10.1007/s00227-020-03770-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-020-03770-2