Abstract
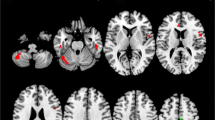
Axonal injury and loss in the corpus callosum (CC) is characteristic of the pathology of multiple sclerosis (MS). Functional magnetic resonance imaging (fMRI) potentially allows neurophysiological consequences of this interhemispheric axonal loss to be defined quantitatively. Here we have used 3T fMRI to study the activation in the contralateral primary sensorimotor cortex and deactivation (mediated by transcallosal tracts) in the homologous ipsilateral region in 14 patients with MS and in 14 matched healthy controls during a simple hand-tapping task. Both healthy controls and MS patients showed similar activation in the motor cortex contralateral to the hand moved, but the patients showed a significantly smaller relative deactivation in the ipsilateral motor cortex (P = 0.002). The difference was accounted for by the sub-group of MS patients who previously had impairment of motor function of the hand tested (MS-phd). The CC of the whole patient group was significantly thinner than for the controls (P = 0.001). Atrophy of the CC was correlated with loss of deactivation for the whole patient group (r = −0.50, P = 0.035), but particularly for MS-phd (r = −0.914, P = 0.004). Interhemispheric physiological inhibition thus is impaired in patients with MS, potentially contributing to impairment of motor control. This work suggests one way in which FMRI monitoring of the transcallosal interactions in motor cortex could become a tool for evaluation of therapies that may enhance function in reversibly impaired pathways.




Similar content being viewed by others
Abbreviations
- EDSS:
-
Expanded disability status scale
- EPI:
-
Echo-planar imaging
- fMRI:
-
Functional MRI
- NART:
-
National adult reading test
- TBV:
-
Total brain volume
- TE:
-
Echo time
- TR:
-
Repetition time
- FOV:
-
Field of view
- MS:
-
Multiple sclerosis
References
Allison JD, Meador KJ, Loring DW, Figueroa RE, Wright JC (2000) Functional MRI cerebral activation and deactivation during finger movement. Neurology 54:135–142
Au Duong MV, Audoin B, Boulanouar K, Ibarrola D, Malikova I, Confort-Gouny S, Celsis P, Pelletier J, Cozzone PJ, Ranjeva JP (2005) Altered functional connectivity related to white matter changes inside the working memory network at the very early stage of MS. J Cereb Blood Flow Metab 25(10):1245–1253
Barkhof FJ, Elton M, Lindeboom J et al (1998) Functional correlates of callosal atrophy in relapsing-remitting multiple sclerosis patients. A preliminary MRI study. J Neurol 245:153–158
Boecker H, Kleinschmidt A, Requardt M, Hanicke W, Merboldt KD, Frahm J (1994) Functional cooperativity of human cortical motor areas during self-paced simple finger movements. A high-resolution MRI study. Brain 117:1231–1239
Boroojerdi B, Diefenbach K, Ferbert A (1996) Transcallosal inhibition in cortical and subcortical cerebral vascular lesions. J Neurol Sci 144:160–170
Boroojerdi B, Hungs M, Mull M, Topper R, Noth J (1998) Interhemispheric inhibition in patients with multiple sclerosis. Electroencephalogr Clin Neurophysiol 109:230–237
Cader S, Cifelli A, Abu-Omar Y, Palace J, Matthews PM (2006) Reduced brain functional reserve and altered functional connectivity in patients with multiple sclerosis. Brain 129(Pt 2):527–37
Comi G, Filippi M, Martinelli V et al (1993) Brain magnetic resonance imaging correlates of cognitive impairment in multiple sclerosis. J Neurol Sci 115(Suppl):S66–S73
Dietemann JL, Beigelman C, Rumbach L et al (1988) Multiple sclerosis and corpus callosum atrophy: relationship of MRI findings to clinical data. Neuroradiology 30:478–480
Evangelou N, Esiri MM, Smith S, Palace J, Matthews PM (2000) Quantitative pathological evidence for axonal loss in normal appearing white matter in multiple sclerosis. Ann Neurol 47:391–395
Gadea M, Marti-Bonmati L, Arana E, Espert R, Casanova V, Pascual A (2002) Dichotic listening and corpus callosum magnetic resonance imaging in relapsing-remitting multiple sclerosis with emphasis on sex differences. Neuropsychology 16:275–281
Ge Y, Law M, Johnson G et al (2004) Preferential occult injury of corpus callosum in multiple sclerosis measured by diffusion tensor imaging. J Magn Reson Imaging 20:1–7
Hamzei F, Dettmers C, Rzanny R, Liepert J, Buchel C, Weiller C (2002) Reduction of excitability (“inhibition”) in the ipsilateral primary motor cortex is mirrored by fMRI signal decreases. Neuroimage 17:490–496
Jenkinson M, Bannister P, Brady M, Smith S (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841
Johansen-Berg H, Rushworth MF, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM (2002) The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci USA 99:14518–14523
Leocani L, Colombo B, Magnani G et al (2001) Fatigue in multiple sclerosis is associated with abnormal cortical activation to voluntary movement—EEG evidence. Neuroimage 13:1186–1192
Meyer BU, Roricht S, Woiciechowsky C (1998) Topography of fibers in the human corpus callosum mediating interhemispheric inhibition between the motor cortices. Ann Neurol 43:360–369
Muller K, Kass-Iliyya F, Reitz M (1997) Ontogeny of ipsilateral corticospinal projections: a developmental study with transcranial magnetic stimulation. Ann Neurol 42:705–711
Newton JM, Sunderland A, Gowland PA (2005) fMRI signal decreases in ipsilateral primary motor cortex during unilateral hand movements are related to duration and side of movement. Neuroimage 24(4):1080–1087
Oh J, Henry RG, Genain C, Nelson SJ, Pelletier D (2004a) Mechanisms of normal appearing corpus callosum injury related to pericallosal T1 lesions in multiple sclerosis using directional diffusion tensor and 1H MRS imaging. J Neurol Neurosurg.Psychiatry 75:1281–1286
Oh J, Pelletier D, Nelson SJ (2004b) Corpus callosum axonal injury in multiple sclerosis measured by proton magnetic resonance spectroscopic imaging. Arch Neurol 61:1081–1086
Pantano P, Mainero C, Iannetti GD et al (2002) Contribution of corticospinal tract damage to cortical motor reorganization after a single clinical attack of multiple sclerosis. Neuroimage 17:1837–1843
Pelletier J, Suchet L, Witjas T et al (2001) A longitudinal study of callosal atrophy and interhemispheric dysfunction in relapsing-remitting multiple sclerosis. Arch Neurol 58:105–111
Petajan JH, White AT (2000) Motor-evoked potentials in response to fatiguing grip exercise in multiple sclerosis patients. Clin Neurophysiol 111:2188–2195
Price CJ, Veltman DJ, Ashburner J, Josephs O, Friston KJ (1999) The critical relationship between the timing of stimulus presentation and data acquisition in blocked designs with fMRI. Neuroimage 10:36–44
Rao SM, Bernardin L, Leo GJ, Ellington L, Ryan SB, Burg LS (1989) Cerebral disconnection in multiple sclerosis. Relationship to atrophy of the corpus callosum. Arch Neurol 46:918–920
Reddy H, Narayanan S, Arnoutelis R et al (2000) Evidence for adaptive functional changes in the cerebral cortex with axonal injury from multiple sclerosis. Brain 123:2314–2320
Rocca MA, Gallo A, Colombo B et al (2004) Pyramidal tract lesions and movement-associated cortical recruitment in patients with MS. Neuroimage 23:141–147
Schmierer K, Niehaus L, Roricht S, Meyer BU (2000) Conduction deficits of callosal fibres in early multiple sclerosis. J Neurol Neurosurg Psychiatry 68:633–638
Schmierer K, Irlbacher K, Grosse P, Roricht S, Meyer BU (2002) Correlates of disability in multiple sclerosis detected by transcranial magnetic stimulation. Neurology 59:1218–1224
Shmuel A, Augath M, Oeltermann A, Logothetis NK (2006) Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci 9:569–577
Smith SM (2002) Fast robust automated brain extraction. Hum Brain Mapp 17:143–155
Stefanovic B, Warnking JM, Pike GB (2004) Hemodynamic and metabolic responses to neuronal inhibition. Neuroimage 22:771–778
Werhahn KJ, Conforto AB, Kadom N, Hallett M, Cohen LG (2003) Contribution of the ipsilateral motor cortex to recovery after chronic stroke. Ann Neurol 54:464–472
Yousry TA, Schmid UD, Alkadhi H et al (1997) Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain 120:141–157
Acknowledgements
S.M. is a trainee in the NIH-Oxford Graduate Partnership Programme. P.M.M. is grateful to the MRC (UK) for personal and for core support of the FMRIB Centre. P.M.M. and J.P. jointly thank the MS Society of Great Britain and Northern Ireland for support of MS studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manson, S.C., Palace, J., Frank, J.A. et al. Loss of interhemispheric inhibition in patients with multiple sclerosis is related to corpus callosum atrophy. Exp Brain Res 174, 728–733 (2006). https://doi.org/10.1007/s00221-006-0517-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-006-0517-4