Abstract
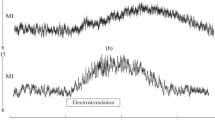
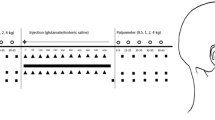
Seeking information on the physiological properties of the trigeminal motoneuronal pool we investigated changes in the excitability of trigeminal motor system induced by two types of experimental pain (muscle and skin). In one session, we studied the effect of muscle pain induced by hypertonic saline infusion into the masseter muscle on the recovery cycle of the heteronymous H-reflex in the temporalis muscle and the homonymous silent period (SP) in the masseter muscle, both elicited by stimulation of the masseteric nerve in ten-healthy subjects. In another session, we studied the effect of laser stimuli applied to the perioral region, at conditioning intervals from 20 to 160 ms, on the temporalis H-reflex and masseter SP in nine healthy subjects. Whereas laser-induced skin pain significantly inhibited the temporalis H-reflex and facilitated the masseter SP (P < 0.01), muscle pain left the time course of the temporalis H-reflex and masseter SP unchanged (P > 0.05). The timing of temporalis H-reflex suppression and masseter-SP enhancement induced by laser stimuli indicates that facial skin nociceptors inhibit trigeminal motoneurones via multysynaptic reflex pathways. Hypertonic saline, a stimulus that predominantly activates group III and IV afferents, left both variables reflecting trigeminal motoneuron excitability unchanged. Due to the differences between the two experimental models, we cannot conclude that such inhibitory reflex pathway does not exist from muscle nociceptors to trigeminal motoneurones.





Similar content being viewed by others
References
Arendt-Nielsen L, Graven-Nielsen T, Svarrer H, Svensson P (1996) The influence of low back pain on muscle activity and coordination during gait: a clinical and experimental study. Pain 64:231–240
Bromm B, Treede RD (1984) Nerve fibre discharges, cerebral potentials and sensations induced by CO2 laser stimulation. Hum Neurobiol 3:33–40
Cairns BE, Svensson P, Wang K, Hupfeld S, Graven-Nielsen T, Sessle BJ, Berde CB, Arendt-Nielsen L (2003) Activation of peripheral NMDA receptors contributes to human pain and rat afferent discharges evoked by injection of glutamate into the masseter muscle. J Neurophysiol 90:2098–2105
Capra NF, Ro JY (2000) Experimental muscle pain produces central modulation of proprioceptive signals arising from jaw muscle spindles. Pain 86:151–162
Cruccu G, Berardelli A, Inghilleri M, Manfredi M (1989) Functional organization of the trigeminal motor system in man. A neurophysiological study. Brain 112:1333–1350
Cruccu G, Romaniello A, Amantini A, Lombardi M, Innocenti P, Manfredi M (1999) Assessment of trigeminal small-fiber function: brain and reflex responses evoked by CO2-laser stimulation. Muscle Nerve 22:508–516
Cruccu G, Truini A, Priori A (2001) Excitability of the human trigeminal motoneuronal pool and interactions with other brainstem reflex pathways. J Physiol 531:559–571
Ellrich J, Hopf HC, Treede RD (1997) Nociceptive masseter inhibitory reflexes evoked by laser radiant heat and electrical stimuli. Brain Res 764:214–220
Godaux E, Desmedt JE (1975) Human masseter muscle: H- and tendon reflexes. Their paradoxical potentiation by muscle vibration. Arch Neurol 32:229–238
Graven-Nielsen T, Svensson P, Arendt-Nielsen L (1997) Effects of experimental muscle pain on muscle activity and co-ordination during static and dynamic motor function. Electroencephalogr Clin Neurophysiol 105:156–164
Iggo A (1961) Non-myelinated afferent fibres from mammalian skeletal muscle. J Physiol 155:52–53
Le Pera D, Graven-Nielsen T, Valeriani M, Oliviero A, Di Lazzaro V, Tonali PA, Arendt-Nielsen L (2001) Inhibition of motor system excitability at cortical and spinal level by tonic muscle pain. Clin Neurophysiol 112:1633–1641
Lorente De Nò R (1933) Vestibulo-ocular reflex arc. Arch Neurol Psychiatry 30:245–291
Lund JP, Donga R, Widmer CG, Stohler CS (1991) The pain-adaptation model: a discussion of the relationship between chronic musculoskeletal pain and motor activity. Can J Physiol Pharmacol 69:683–694
Macaluso GM, De Laat A (1995) H-reflexes in masseter and temporalis muscles in man. Exp Brain Res 107:315–320
Macaluso GM, Graven-Nielsen T, Svensson P (2001) Conditioning of heteronymous H reflex in human temporalis muscle by stimulation of perioral afferents. Exp Brain Res 136:114–119
Masri R, Ro JY, Capra N (2005) The effect of experimental muscle pain on the amplitude and velocity sensitivity of jaw closing muscle spindle afferents. Brain Res 1050:138–147
Matre DA, Sinkjaer T, Svensson P, Arendt-Nielsen L (1998) Experimental muscle pain increases the human stretch reflex. Pain 75:331–339
Mense S (1993) Nociception from skeletal muscle in relation to clinical muscle pain. Pain 54:241–289
Nakamura Y (1980) Brainstem neuronal mechanisms controlling the trigeminal motoneuron activity. In: Desmedt JE (ed) Spinal and supraspinal mechanisms of voluntary motor control and locomotion. Progress in Clinical Neurophysiology, vol. 8. Karger, Basel, pp 181–202
Ro JY, Capra NF (1999) Evidence for subnucleus interpolaris in craniofacial muscle pain mechanisms demonstrated by intramuscular injections with hypertonic saline. Brain Res 842:166–183
Romaniello A, Cruccu G, McMillan AS, Arendt-Nielsen L, Svensson P (2000) Effect of experimental pain from trigeminal muscle and skin on motor cortex excitability in humans. Brain Res 882:120–127
Romaniello A, Arendt-Nielsen L, Cruccu G, Svensson P (2002) Modulation of trigeminal laser evoked potentials and laser silent periods by homotopical experimental pain. Pain 98:217–228
Rossi A, Mazzocchio R, Decchi B (2003) Effect of chemically activated fine muscle afferents on spinal recurrent inhibition in humans. Clin Neurophysiol 114:279–287
Sessle BJ (1991) Physiology of the trigeminal system. In: Fromm GH, Sessle BJ (eds) Trigeminal Neuralgia: Current Concepts Regarding Pathogenesis and Treatment. Butterworth-Heinemann, Boston, pp 71–105
Svensson P, Arendt-Nielsen L, Houe L (1996) Sensory-motor interactions of human experimental unilateral jaw muscle pain: a quantitative analysis. Pain 64:241–249
Svensson P, Houe L, Arendt-Nielsen L (1997) Bilateral experimental muscle pain changes electromyographic activity of human jaw-closing muscles during mastication. Exp Brain Res 116:182–185
Svensson P, Arendt-Nielsen L, Houe L (1998a) Muscle pain modulates mastication: an experimental study in humans. J Orofac Pain 12:7–16
Svensson P, De Laat A, Graven-Nielsen T, Arendt-Nielsen L (1998b) Experimental jaw-muscle pain does not change heteronymous H-reflexes in the human temporalis muscle. Exp Brain Res 121:311–318
Svensson P, De Laat A, Graven-Nielsen T, Arendt-Nielsen L, Macaluso GM (1999) R Effect of clenching levels on heteronymous H-reflex in human temporalis muscle. Exp Brain Res 126:467–472
Svensson P, Miles TS, McKay D, Ridding MC (2003) Suppression of motor evoked potentials in a hand muscle following prolonged painful stimulation. Eur J Pain 7:55–62
Treede RD (2003) Neurophysiological studies of pain pathways in peripheral and central nervous system disorders. J Neurol 250:1152–1161
Treede RD, Meyer RA, Raja SN, Campbell JN (1995) Evidence for two different heat transduction mechanisms in nociceptive primary afferents innervating monkey skin. J Physiol 483:747–758
Wang K, Svensson P, Arendt-Nielsen L (2000) Effect of tonic muscle pain on short-latency jaw-stretch reflexes in humans. Pain 88:189–197
Wang K, Arendt-Nielsen L, Svensson P (2001) Excitatory actions of experimental muscle pain on early and late components of human jaw stretch reflexes. Arch Oral Biol 46:433–442
Westberg KG, Clavelou P, Schwartz G, Lund JP (1997) Effects of chemical stimulation of masseter muscle nociceptors on trigeminal motoneuron and interneuron activities during fictive mastication in the rabbit. Pain 73:295–308
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Truini, A., Romaniello, A., Svensson, P. et al. Experimental skin pain and muscle pain induce distinct changes in human trigeminal motoneuronal excitability. Exp Brain Res 174, 622–629 (2006). https://doi.org/10.1007/s00221-006-0508-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-006-0508-5