Abstract
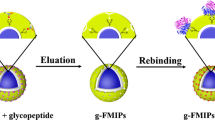
A novel and facile fluorescent artificial receptor on the basis of the molecularly imprinted polymer-coated graphene quantum dots was engineered successfully to detect colistin. The colistin imprinted graphene quantum dots (CMIP-GQDs) was synthesized by vinyl-based radical polymerization between functional monomers and crosslinker at around the template molecule on the surface of graphene quantum dots. The size of bare, CNIP-GQDs, and CMIP-GQDs was about 4.8 ± 0.6 nm, 18.4 ± 1.7 nm, and 19.7 ± 1.3 nm, respectively. The CMIP-GQDs, which showed the strong fluorescence emission at 440 nm with the excitation wavelength fixed at 380 nm, had excellent selectivity and specificity to rapidly recognize and detect colistin. The linear range of fluorescence quenching of this fluorescent artificial receptor for detection colistin was 0.016–2.0 μg mL−1 with a correlation coefficient (R2) of 0.99919, and the detection limit was 7.3 ng mL−1 in human serum samples. The designed receptor was successfully applied to detect colistin in human serum samples and it achieved excellent recoveries shifted from 93.8 to 105%.

Graphical abstract













Similar content being viewed by others
References
Ma Z, Wang J, Nation RL, Li J, Turnidge JD, Coulthard K, et al. Renal disposition of colisitin in the isolated perfused rat kidney. Antimicrob Agents Chemother. 2009;53:2857–64. https://doi.org/10.1128/AAC.00030-09.
Bergen PJ, Li J, Rayner CR, Nation RL. Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2006;50:1953–8. https://doi.org/10.1128/AAC.00035-06.
Pogue JM, Lee J, Marchaim D, Yee V, Zhao JJ, Chopra T, et al. Incidence of and risk factors for colistin- associated nephrotoxicity in a large academic health system. Clin Infect Dis. 2011;53:879–84. https://doi.org/10.1093/cid/cir611.
Yousef JM, Chen G, Hill PA, Nation RL, Li J. Ascorbic acid protectts against the nephrotoxicity and apoptosis caused by colistin and affects its pharmokinetics. J Antimicrob Chemother. 2012;67:452–9. https://doi.org/10.1093/jac/dkr483.
Nation RL, Garonzik SM, Thamlikitkul V, Giamarellos-Bourboulis EJ, Forrest A, Paterson DL, et al. Dosing guidance for intravenous colistin in critically-ill patients. Clin Infect Dis. 2017;64:565–71. https://doi.org/10.1093/cid/ciw839.
Antachopoulos C, Karvanen M, Iosifidis E, Jansson B, Plachouras D, Cars O, et al. Serum and cerebrospinal fluid levels of colistin. Antimicrob Agents Chemother. 2010;54:3985–7. https://doi.org/10.1128/AAC.01799-09.
Gai Z, Samodelov SL, Kullak-Ublick GA, Visentin M. Molecular mechanisms of colistin-induced nephrotoxicity. Molecules. 2019;24:653–66. https://doi.org/10.3390/molecules24030653.
Turan E, Şahin F. Molecularly imprinted biocompatible magnetic nanoparticles for specific recognition of Ochratoxin A. Sens Actuators B Chem. 2016;227:668–76. https://doi.org/10.1016/j.snb.2015.12.087.
Scheller FW, Zhang X, Yarman A, Wollenberg U, Gyurcsanyi RE. Molecularly imprinted polymer-based electrochemical sensors for biopolymers. Curr Opin Electrochem. 2019;14:53–9. https://doi.org/10.1016/j.coelec.2018.12.005.
Turan E. His-tag-epitope imprinted thermoresponsive magnetic nanoparticles for recognition and separation thyroid peroxidase antigens from whole blood samples. ChemSelect. 2018;3:11963–9. https://doi.org/10.1002/slct.201801557.
BelBruno JJ. Molecularly imprinted polymers. Chem Rev. 2019;119:94–119. https://doi.org/10.1021/acs.chemrev.8b00171.
Pan J, Chen W, Ma Y, Pan G. Molecularly imprinted polymers as receptor mimics for selective cell recognition. Chem Soc Rev. 2018;47:5574–87. https://doi.org/10.1039/c7cs000854f.
Choi JR, Yong YW, Choi JY, Cowie AC. Progress in molecularly imprinted polymers for biomedical applications. Comb Chem High Throughput Screen. 2019;22:78–88. https://doi.org/10.2174/1386207322666190325115526.
Xu J, Miao H, Wang J, Pan G. Molecularly imprinted synthetic antibodies: from chemical design to biomedical applications. NANO MICRO Small. 2020;1906644:1–21. https://doi.org/10.1002/smll.201906644.
Ensafi AA, Zakery M, Rezai B. An optical sensor with specific binding sites for the detection of thioridazine hydrochloride based ZnO-QDs coated with molecularly imprinted polymer. Spectrochim Acta A Mol Biomol Spectrosc. 2019;206:460–5. https://doi.org/10.1016/j.saa.2018.08.040.
Yan YY, He XW, Li WY, Zhang YK. Nitrogen-doped graphene quantum dots-labeled epitope imprinted polymer with double templates via the metal chelation for specific recognition of cytochrome c. Biosens Bioelectron. 2017;91:253–61. https://doi.org/10.1016/j.bios.2016.12.040.
Khataee A, Hassanzadeh J, Kohen E. Specific quantification of atropine using molecularly imprinted polymer on graphene quantum dots. Spectrochim Acta A Mol Biomol Spectrosc. 2018;205:614–21. https://doi.org/10.1016/j.saa.2018.07.088.
Liu Y, Cao N, Gui W, Ma Q. Nitrogen-doped graphene quantum dots-based fluorescence molecularly imprinted sensor for thiacloprid detection. Talanta. 2018;183:339–44. https://doi.org/10.1016/j.talanta.2018.01.063.
Toloza CAT, Almeida JMS, Khan S, dos Santos YG, da Silva AR, Aucelio RQ. Kanamycin detection at graphene quantum dot-decorated gold nanoparticles in organized medium after solid-phase extraction using an aminoglycoside imprinted polymer. MethodsX. 2018;5:1605–12. https://doi.org/10.1016/j.mex.2018.11.019.
Chullasat K, Kanatharana P, Bunkoed O. Nanocomposite optosensor of dual quantum dot fluorescence probes for simultaneous detection of cephalexin and ceftriaxone. Sens Actuators B Chem. 2019;281:689–97. https://doi.org/10.1016/j.snb.2018.11.003.
Mehrzad-Samarin M, Faridbod F, Ganjali MR. A luminescence nanosensor for ornidazole detection using graphene quantum dots entrapped in silica molecular imprinted polymer. Spectrochim Acta A Mol Biomol Spectrosc. 2019;206:430–6. https://doi.org/10.1016/j.saa.2018.08.026.
Li Y, Tang S, Zhang W, Cui X, Zhang Y, Jin Y, et al. A surface-enhanced Raman scattering-based lateral flow immunosensor for colistin in raw milk. Sens Actuators B Chem. 2019;282:703–11. https://doi.org/10.1016/j.snb.2018.11.050.
Suhren G, Knappstein K. Detection of colistin in spiked and incurred milk samples by LC- and ELISA-technique. Anal Chim Acta. 2005;529:97–101. https://doi.org/10.1016/j.aca.2004.09.030.
Yuphintharakum N, Nurerk P, Chullasat K, Kanatharana P, Davis F, Sooksawat D, et al. A nanocomposite optosensor containing carboxylic functionalized multiwall carbon nanotubes and quantum dots incorporated into a molecularly imprinted polymer for highly selective and sensitive detection of ciprofloxacin. Spectrochim Acta A Mol Biomol Spectrosc. 2018;201:382–91. https://doi.org/10.1016/j.saa.2018.05.034.
Zhang L, Chen L. Fluorescence probe based on hybrid mesoporous silica/quantum dot/molecularly imprinted polymer for detection of tetracycline. ACS Appl Mater Interfaces. 2016;8:16248–56. https://doi.org/10.1021/acsami.6b04381.
Lu X, Wei F, Xu G, Wu Y, Yang J, Hu Q. Surface molecular imprinting on silica coated CdTe quantum dots for selective and sensitive fluorescence detection of p-aminophenol in water. J Fluoresc. 2017;27:181–9. https://doi.org/10.1007/s10895-016-1944-7.
Gao T, Wang X, Yang LY, He H, Ba XX, Zhao J, et al. Red, yellow, and blue luminescence by graphene quantum dots: synthesis, mechanism, and cellular imaging. ACS Appl Mater Interfaces. 2017;9:24846–56. https://doi.org/10.1021/acsami.7b05569.
Xiao Y, Xiao R, Tang J, Zhu Q, Li X, Xiong Y, et al. Preparation and adsorption properties of molecularly imprinted polymer via RAFT precipitation polymerization for selective removal of aristolochic acid I. Talanta. 2017;162:415–22. https://doi.org/10.1016/j.talanta.2016.10.014.
Chullasat K, Nurerk P, Kanatharana P, Davis F, Bunkoed O. A facile optosensing protocol based on molecularly imprinted polymer coated on CdTe quantum dots for highly sensitive and selective amoxicillin. Sens Actuators B Chem. 2018;254:255–63. https://doi.org/10.1016/j.snb.2017.07.062.
Zhou Z, Li T, Xu W, Huang W, Wang N, Yang W. Synthesis and charazterization of fluorescence molecularly imprinted polymers as sensor for highly sensitive detection of dibutyl phthalate from tap water samples. Sens Actuators B Chem. 2017;204:1114–22. https://doi.org/10.1016/j.snb.2016.09.092.
Geng S, Lin SM, Li NB, Luo HQ. Polyethylene glycol capped ZnO quantum dots as a fluorescent probe for determining copper (II) ion. Sens Actuators B Chem. 2017;253:137–43. https://doi.org/10.1016/j.snb.2017.06.118.
Ren X, Chen L. Preparation of molecularly imprinted polymer coated quantum dots to detect nicosulfuron in water samples. Anal Bioanal Chem. 2015;407:8087–95. https://doi.org/10.1007/s00216-015-8982-x.
Zhang L, Chen L. Visual detection of melamine by using a ratiometric fluorescent probe consisting of a red emitting CdTe core and a green emitting CdTe shell coated with a molecularly imprinted polymer. Microchim Acta. 2018;185:135–43. https://doi.org/10.1007/s00604-017-2664-7.
Orachorn N, Bunkoed O. A nanocomposite fluorescent probe of polyaniline, graphene oxide and quantum dots incorporated into highly selective polymer for lomefloxacin detection. Talanta. 2019;203:261–8. https://doi.org/10.1016/j.talanta.2019.05.082.
Raksawong P, Nurerk P, Chullasat K, Kanatharana P, Bunkoed O. A polypyrolle doped with fluorescent CdTe quantum dots and incorporated into molecularly imprinted silica for fluorometric determination of ampicillin. Microchim Acta. 2019;186:1–8. https://doi.org/10.1007/s00604-019-3447-0.
Ahmadpour H, Hosseini SMM. A solid-phase luminescence sensor based on molecularly imprinted polymer-CdSeS/ZnS quantum dots for selective extraction and detection of sulfasalazine in biological samples. Talanta. 2019;194:534–41. https://doi.org/10.1016/j.talanta.2018.10.053.
Chepyala D, Tsai IL, Sun HY, Lin SW, Kuo CH. Development and validation of a high-performance liquid chromatography-fluorescence detection method for the accurate qantification of colistin in human plasma. J Chromatogr B. 2015;980:48–54. https://doi.org/10.1016/j.jchromb.2014.12.015.
Cangemi G, Barco S, Castagnola E, Tripodi G, Favata F. Development and validation of UHPLC-MS/MS methods for the quantification of colistin in plasma and dried plasma spots. J Pharm Biomed Anal. 2016;129:551–7. https://doi.org/10.1016/j.jpba.2016.08.002.
Zhao M, Wu XJ, Fan YX, Guo BN, Zhang J. Development and validation of a UHPLC-MS/MS assay for colistin methanesulphonate (CMS) and colistin in human plasma and urine using weak-cation exchange solid-phase extraction. J Pharm Biomed Anal. 2016;124:303–8. https://doi.org/10.1016/j.jpba.2016.02.045.
Boison JO, Lee S, Matus J. A multi residue method for the determination of seven polypeptide drug residues in chicken muscle tissues by LC-MS/MS. Anal Bioanal Chem. 2015;407:4065–78. https://doi.org/10.1007/s00216-015-8644-z.
Gobin P, Lemaitre F, Marchand S, Couet W, Olivier JC. Assay of colistin and colistin methanesulfonate in plasma and urine by liquid chromatography-tandem mass spectrometry. Antimicrob Agents Chemother. 2010;54:1941–8. https://doi.org/10.1128/AAC.01367-09.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were approved by Istanbul Medipol University Non-invasive Clinical Research Ethics Committee, Turkey, and in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Turan, E., Zengin, A. A fluorescent artificial receptor with specific imprinted cavities to selectively detect colistin. Anal Bioanal Chem 412, 7417–7428 (2020). https://doi.org/10.1007/s00216-020-02873-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02873-5